Why Friedel Craft's Methylation of Toluene is abnormal?
At low temperatures (sub-zero), toluene reacts with chloro-methane with catalyst anhydrous aluminum chloride, which gives ortho-xylene (54%) as the major product while at room temperature or higher, this reaction provides meta-xylene (89%) as the major product even though methyl group is ortho-para directing group. Then why did it happen so? Could you provide your explanation for this phenomenon?
Reaction Diagram of Friedel-Craft Methylation of Toluene and Concept of KCP and TCP:
Before discussing the reason for abnormalities, it is important to understand the reaction diagram and concept of KCP and TCP for the Friedel-craft reaction of toluene at different temperatures. So it will help to create a knowledge base for your easy understanding in further explanation.
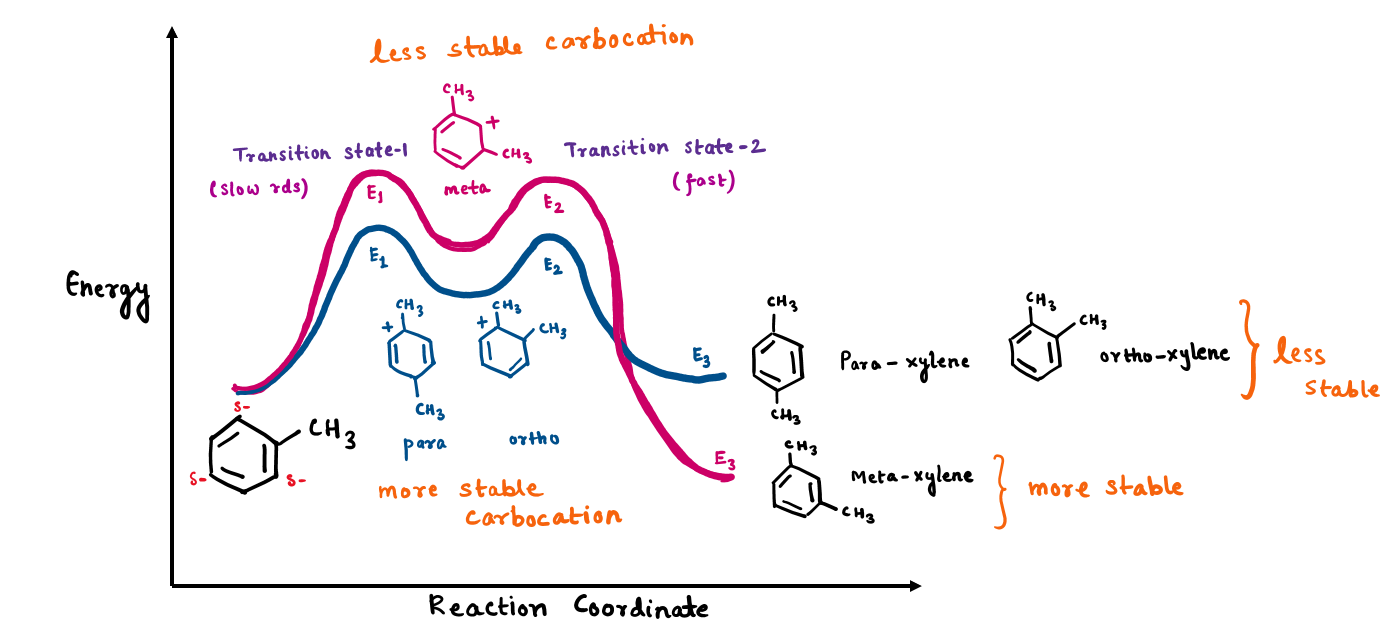
Friedel-reaction alkylation (FCA) of toluene proceeds through a two-step mechanism which has its own transition state with two peaks
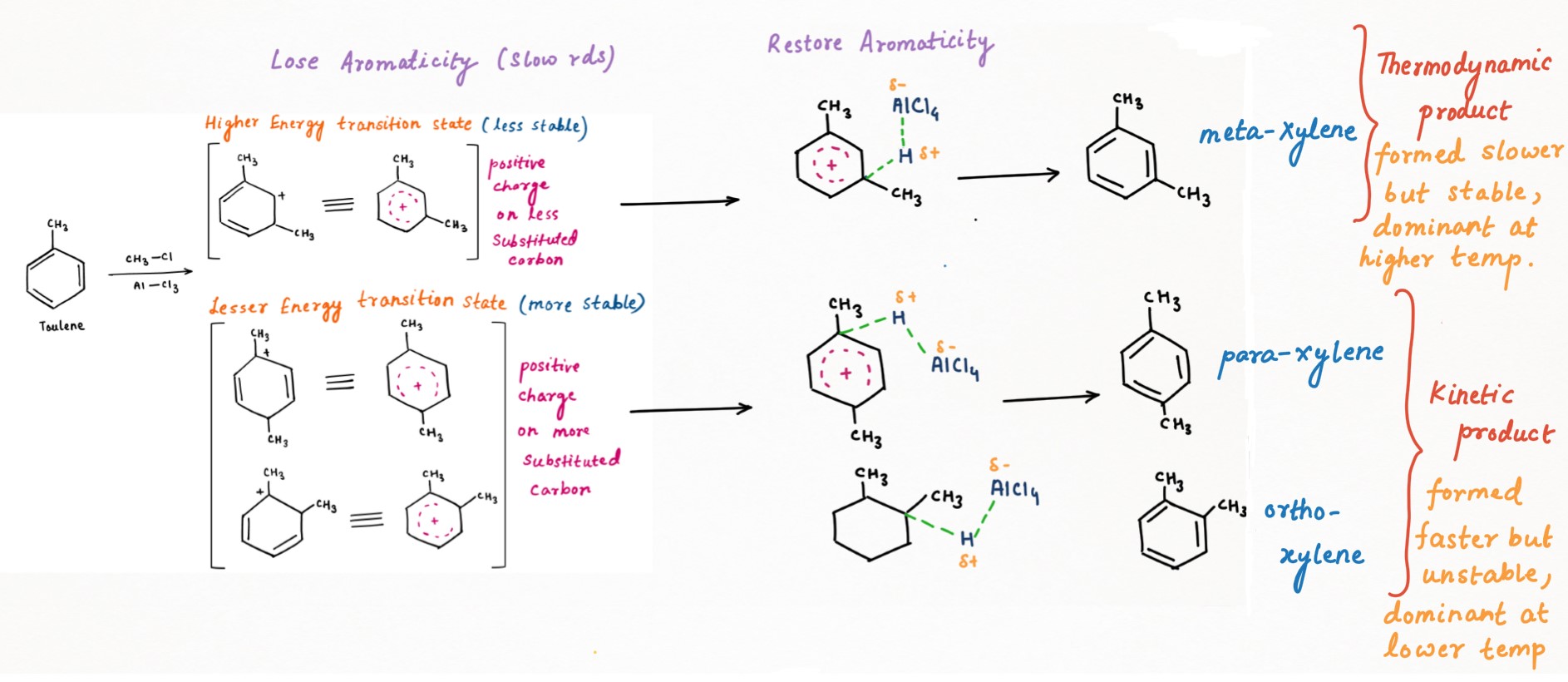
- In the first step, toluene acting as a nucleophile, attacks on polarized-complex electrophile (complex formation by AlCl3 and chloromethane). This is a slow and rate-determining step (RDS) as it loses aromaticity and leads to the formation of carbocation.
- In a second step (fast), C-H bond is broken to deprotonate and restore aromaticity by re-forming C-C pi bond.
Since relative heights of the peaks should be the rate-limiting step, this means the transition state with the highest activation energy ( E1 in this case) will be slow and the rate-determining step (RDS). We will study the concept of kinetic and thermodynamically controlled products ( TCP and KCP) concerning the energy of RDS. Temperature plays a big role in deciding kinetic or thermodynamic products.
Let's introduce a new mathematical perception (ΔE), to evaluate the spontaneity or speed of reaction, that which product will form faster or slower.
ΔE = E1-E3
= Energy of RDS - Energy of product
A product that is easier to form with the lowest required energy ΔE is called a kinetic-controlled product (KCP) and a reaction is called a kinetic product (KC). Hence, it will be applicable at a lower temperature as KCP will be the dominant & major product in the mixture.
In simple words, you can decide on KCP by checking the height of energy of RDS (E1). The product with the lowest RDS energy (E1) will be a kinetic product or you can also say that an intermediate with high stability will have the lowest RDS energy, and hence it will be a kinetic product.
However, the concept of ΔE becomes irrelevant for thermodynamic products at higher temperatures. Because at high temperatures, there is high enough energy to form many products, and the reaction to form products is reversible.
Hence, a product that is stable with the lowest E3 energy is called a thermodynamic-controlled product (TCP) and a reaction is called thermodynamic control. So, thermodynamic products will be a major and dominant product at higher temperatures. It is decided by the stability of the product, not the intermediate.
Let's summarize in brief. The kinetic product is easier to form and dominant at lower temperatures but the thermodynamic product is more stable, formed slowly, and will dominate at higher temperatures.
Concept of KCP and TCP for Friedel-Craft Methylation of Toluene:
As we have learned the general concept of KCP and TCP in the previous section, it is easy for us to discuss this same concept for Friedel-craft methylation of toluene. For kinetic, it depends on ΔE (difference of E1 and E3) or the height of energy of RDS (E1).
From above reaction diagram, Energy of RDS (E1): meta> ortho and para (Stability of intermediate [RDS]: ortho and para> meta)
Now coming to ΔE, ΔE for meta will be higher than for ortho and para. Hence, ortho and para-xylene will be easier to form at lower temperatures and will be kinetic products (major).
For thermodynamic products, it is decided by stability of the product or the height of product energy (E3).
From above reaction diagram, Energy of product (E3): ortho and para> meta (Stability of product: meta> ortho and para). Hence, meta-xylene will be a thermodynamic product (major).
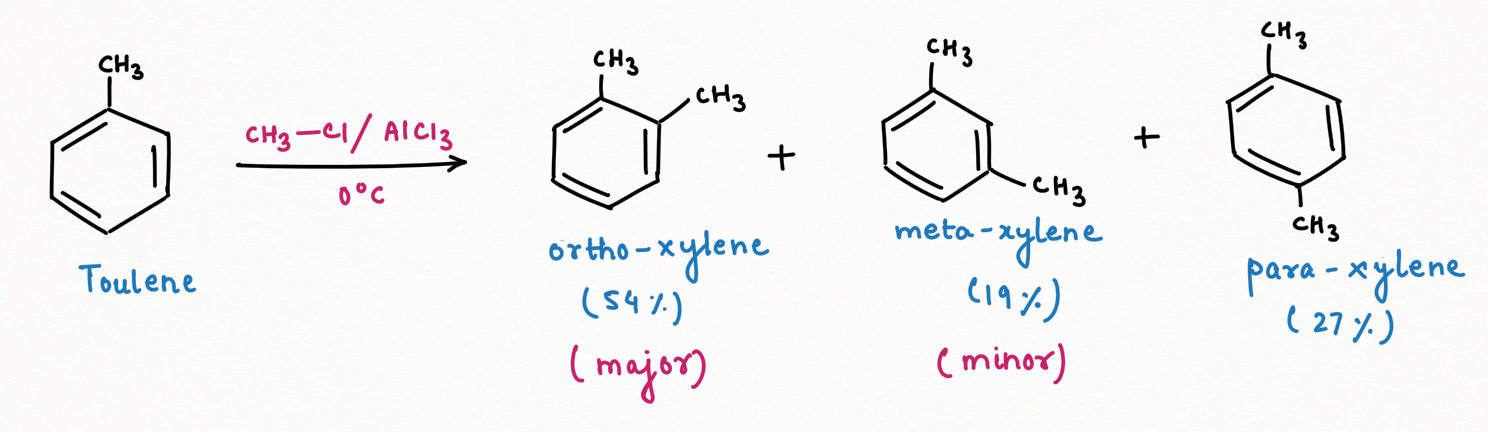
At sub-zero temperatures, a kinetic product will be formed as a major. So, Friedel-Craft Alkylation of toluene gives ortho-xylene (54%) as a major product, followed by para-xylene (28%), and then meta-xylene (19%) as a minor product.
Friedel-Craft Methylation of Toluene at Various Temperatures:
At room temperature (298 K), a thermodynamic product will be formed as a major and the most stable. So, Friedel-Craft Alkylation of toluene gives meta-xylene (69%) as a major product, followed by para-xylene (28%), and then ortho-xylene (3%) as a minor product.
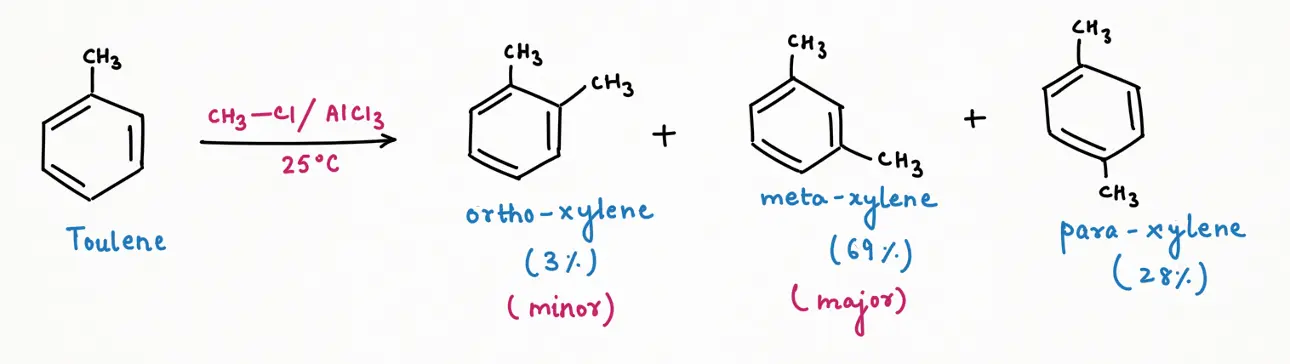
At higher temperature (let's take 80 degree Celsius), a thermodynamic product will be formed as a major and the most stable. So, Friedel-Craft Alkylation of toluene gives meta-xylene (89%) as a major product, followed by para-xylene (10%), and then ortho-xylene (1%) as a minor product at higher temperature.
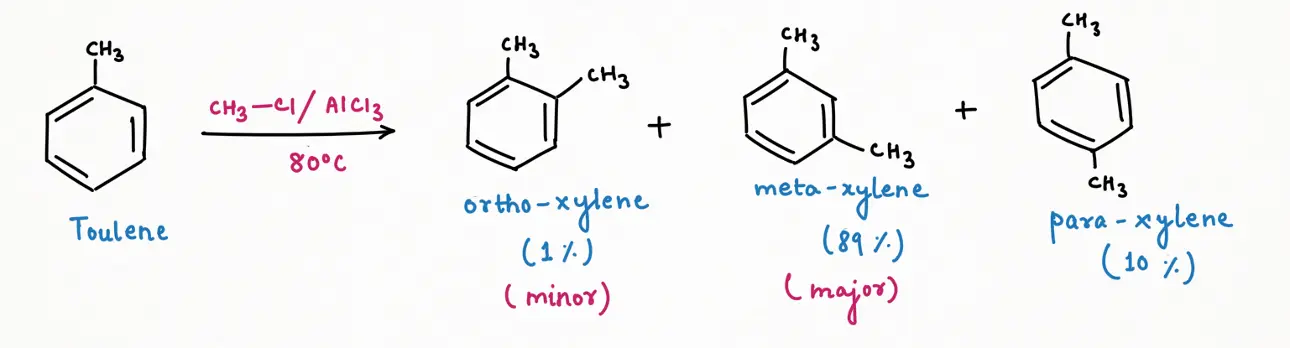
From the above three reactions, we can predict a trend of mixture composition very easily. As we increase the temperature of the reaction, the percent composition of meta-xylene will increase while their isomer para and ortho-xylene will decrease. Temperature plays a very important role in changing mixture composition.
Why Friedel-Craft Methylation of Toluene is Abnormal?
After intensive discussion of the reaction diagram, basics of KCP and TCP, and mixture composition of xylene's isomer at different temperatures, finally came to understand the reason for this abnormality which we have been waiting for. There are three reasons why meta-xylene is a major product even though methyl group is an o-p directing influencer due to the nature of electron-releasing nature which we expect ortho-para xylene as a major but this is not expected product as we have seen already in the above reaction and experiments.
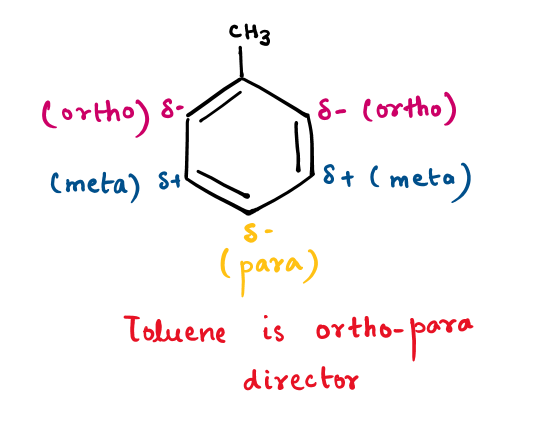
First Reason: Why Meta-Xylene is considered a stable product resulting in major and thermodynamic products at a higher temperature while the rest isomer of xylene doesn't?
In aromatic chemistry, the attachment of a methyl group to the sp2 carbon of benzene initiates hyperconjugation, influencing the stability and reactivity of the resulting compound. This phenomenon is particularly notable in the various isomers of xylene: ortho-xylene, meta-xylene, and para-xylene.
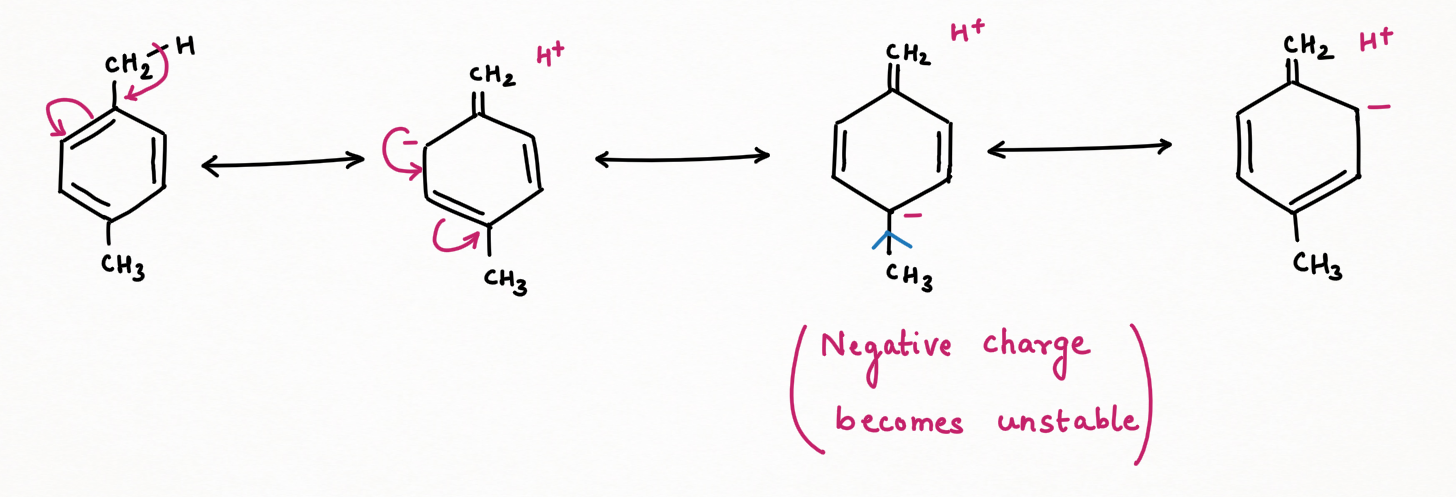
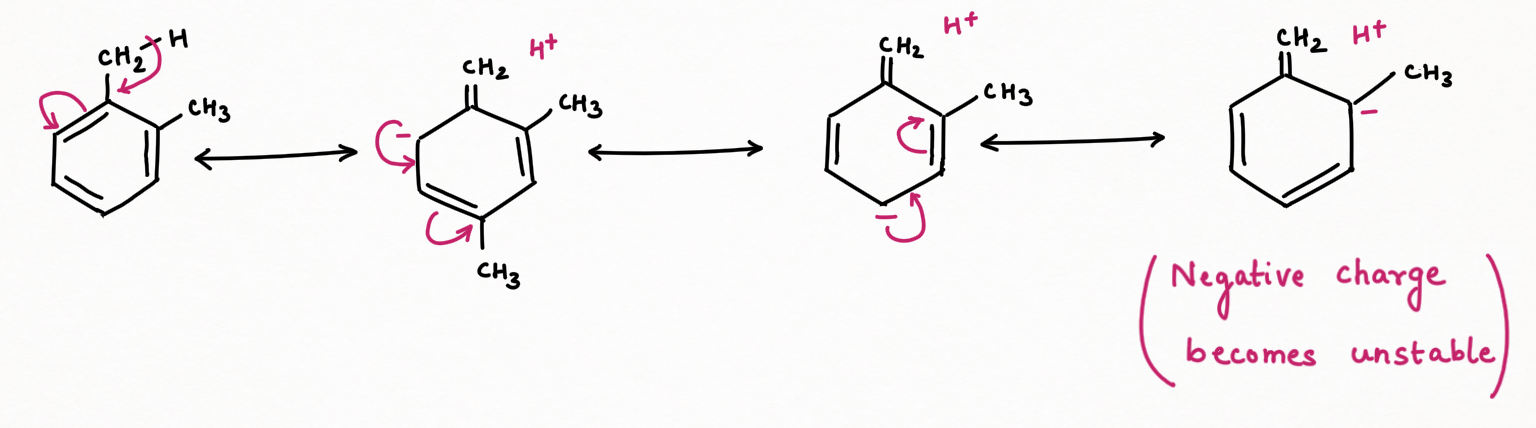
Due to the o-p-directing nature of the methyl group, when the negative charge is delocalized at ortho or para-position to which another methyl is substituted to form ortho or para-xylene respectively, then this charge will become more unstable due to the electron-releasing nature of the second methyl.
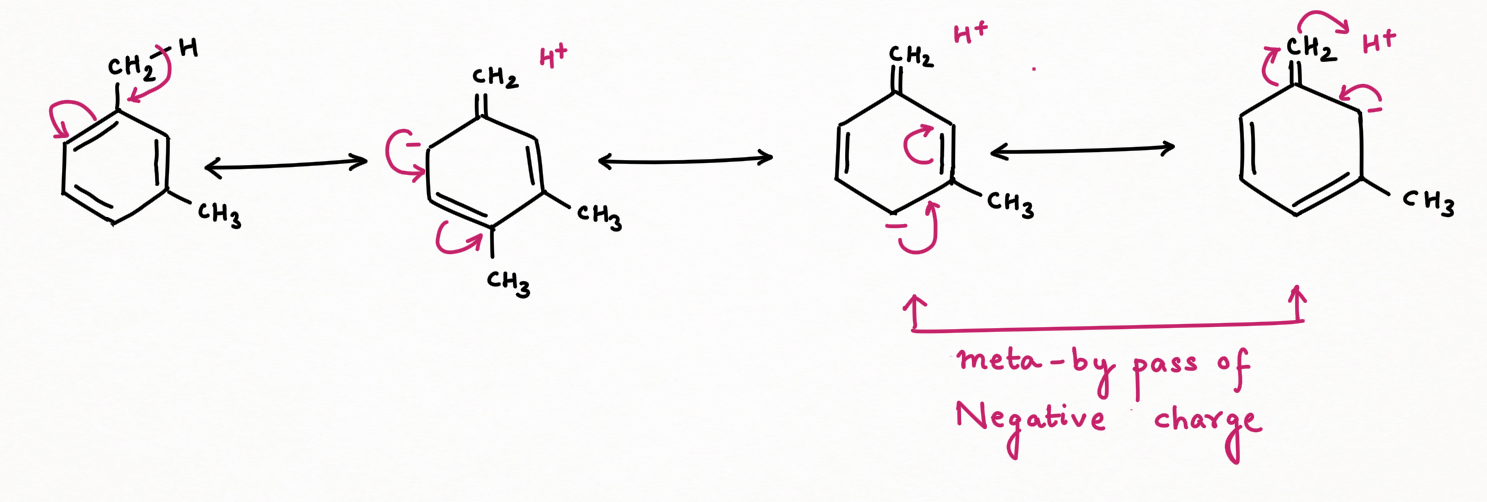
Conversely, in meta-xylene, the negative charge delocalized through resonance does not localize at the meta position. Instead, it bypasses this position, avoiding the destabilizing interaction that occurs in the ortho and para isomers. As a result, the negative charge is more stable in meta-xylene. Hence it is considered more stable than para-ortho xylene and will be a thermodynamic and major product at higher temperatures.
Second Reason: Dynamic equilibrium between ortho or para-xylene and meta-xylene via isomerization and dealkylation
Recent research has revealed a fascinating dynamic equilibrium between ortho-xylene, para-xylene, and meta-xylene, shedding light on their interconversion mechanisms and stability.
A notable study by Scientist March has demonstrated that, over prolonged reaction times ranging from 5 to 33 hours, a dynamic equilibrium is established between ortho or para-xylene and meta-xylene. The forward reaction, favoring the formation of meta-xylene, dominates this equilibrium, leading to a greater accumulation of meta-xylene (Kf > Kb).
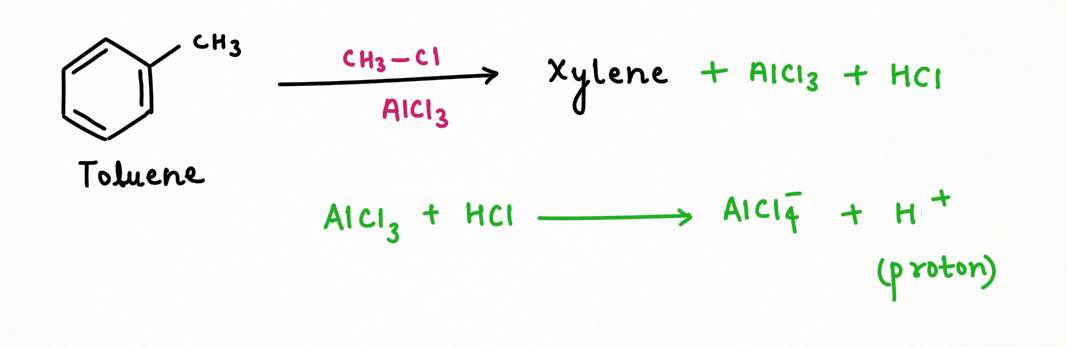
This equilibrium is explained by the processes of isomerization and dealkylation, facilitated by the use of a mixture of side products, specifically HCl and anhydrous AlCl3. This combination has a remarkable ability to generate protons, which are crucial in the protonation of benzene derivatives. Through protonation, methyl groups are shifted, enabling the conversion of ortho- and para-xylene into the more stable meta-xylene.
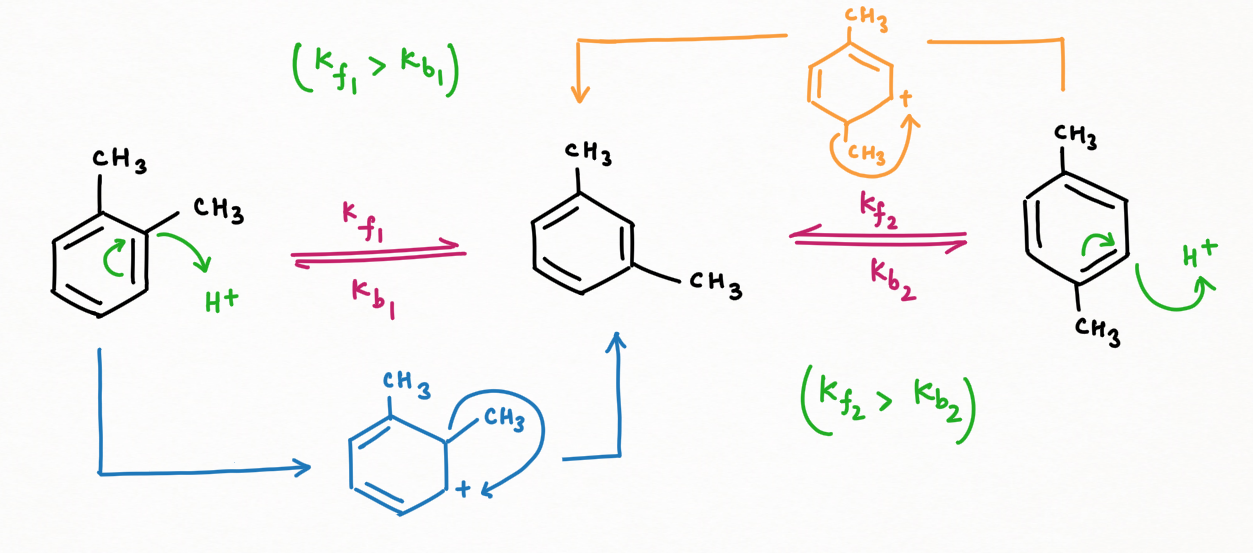
The research highlights the potential of HCl and anhydrous AlCl3 to drive this transformation, emphasizing their role in creating a dynamic system where meta-xylene is the predominant product over time.
By studying the dynamic equilibrium and isomerization of xylene isomers, we better understand the chemical processes, ultimately favoring the formation of the thermodynamically stable meta-xylene.
Bonus point: What will happen if chloromethane is given in excess in the Friedel-craft reaction of toluene? Will there be any different or just the same scenario?
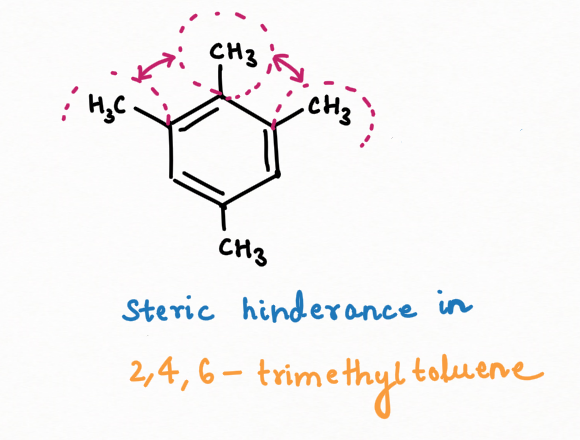
When chloromethane is used in excess in the Friedel-Crafts reaction of toluene, the reaction does not stop at the formation of xylene but continues to produce compounds with more than two methyl substituents. At lower temperatures, 2,4,5-trimethyl toluene is the major kinetic product, while mesitylene becomes the dominant thermodynamic product at room or higher temperatures.
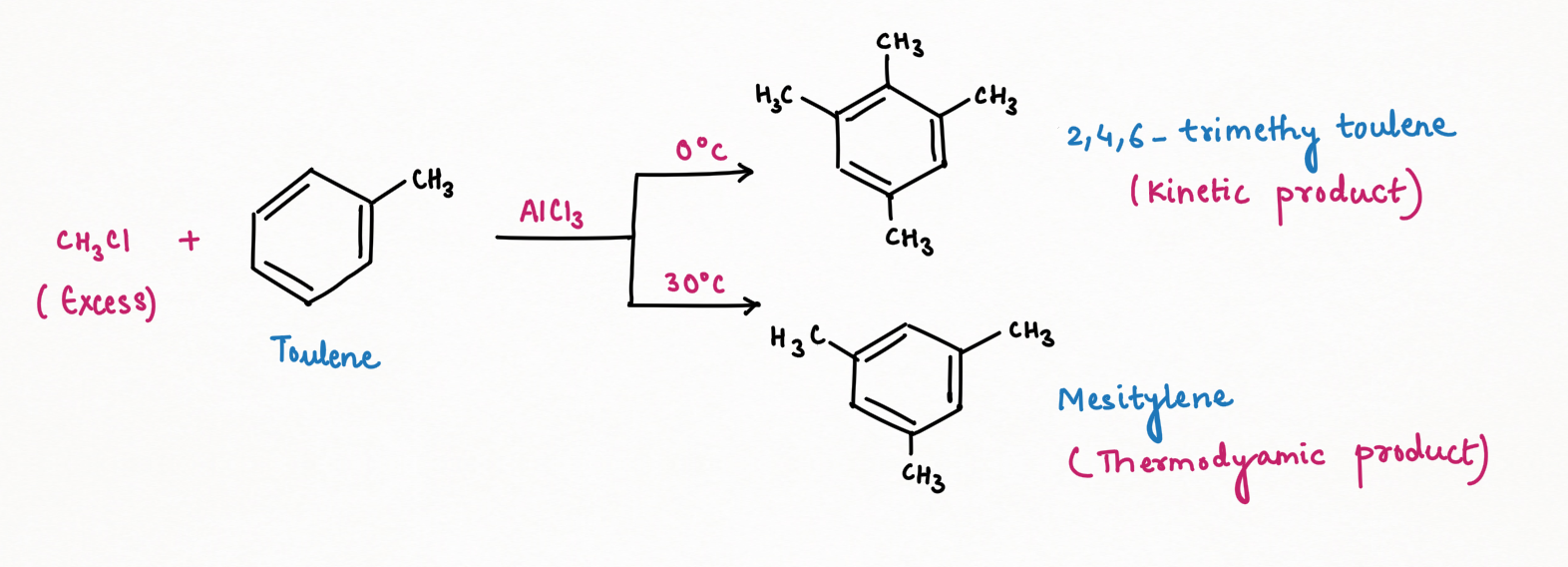
This difference in products highlights the relative stability of 2,4,5-trimethyl toluene and mesitylene. The kinetic product, 2,4,5-trimethyl toluene, is less stable due to significant steric hindrance and van der Waals repulsion between the methyl groups. In contrast, mesitylene is more stable because the negative charge is delocalized, bypassing the meta position, and reducing electron repulsion.
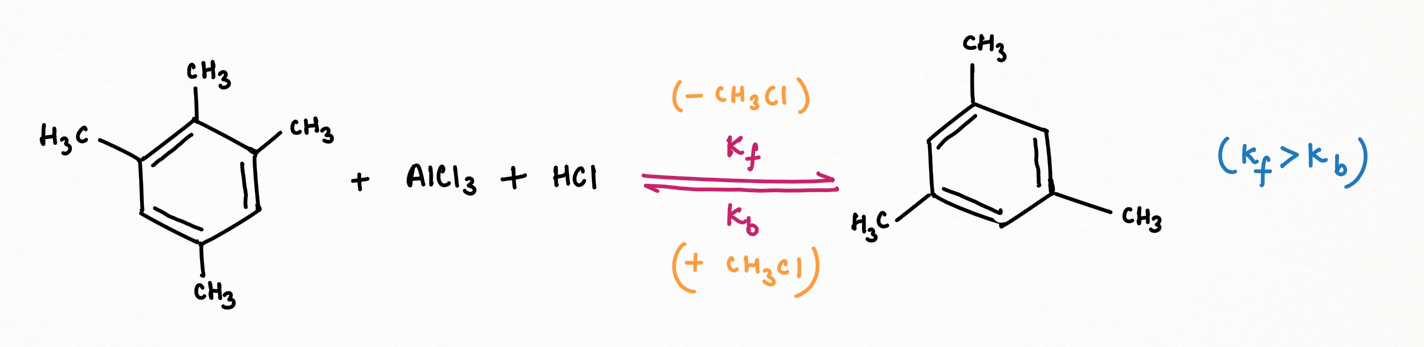
Again, according to Scientist March, in this case, has revealed an equilibrium between 2,4,5-trimethyl toluene and mesitylene. This equilibrium involves the dealkylation of one methyl group from 2,4,5-trimethyl toluene, resulting in the formation of the more stable mesitylene. The forward reaction dominates this equilibrium, favoring the accumulation of mesitylene (Kf > Kb), with the byproducts of chloromethane, HCl, and anhydrous AlCl3.
Conclusion :
In this brain teaser question, I discuss the abnormal Friedel-Crafts methylation of toluene, where the major product varies with temperature. At sub-zero temperatures, ortho-xylene is the major product, while at room and higher temperatures, meta-xylene becomes predominant despite methyl groups being ortho-para directing. This phenomenon is explained by kinetic and thermodynamic control. Excess chloromethane leads to further alkylation, forming products like 2,4,5-trimethyl toluene (kinetic product) and mesitylene (thermodynamic product). An equilibrium between these products is established via isomerization and dealkylation.
Big Thanks to you for reading and diving deep into the interesting world of xylene isomers. Keep Informed!! Be curious, experiment and dive deep into this magic of organic reactions. Thanks for reading, and happy learning!
For more intriguing chemistry insights, visit Science Mania.