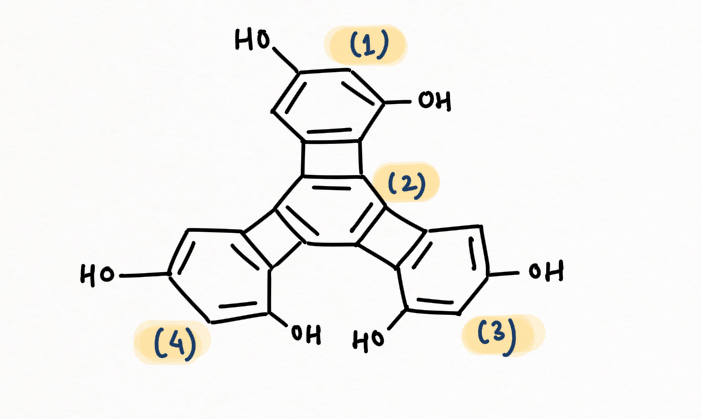
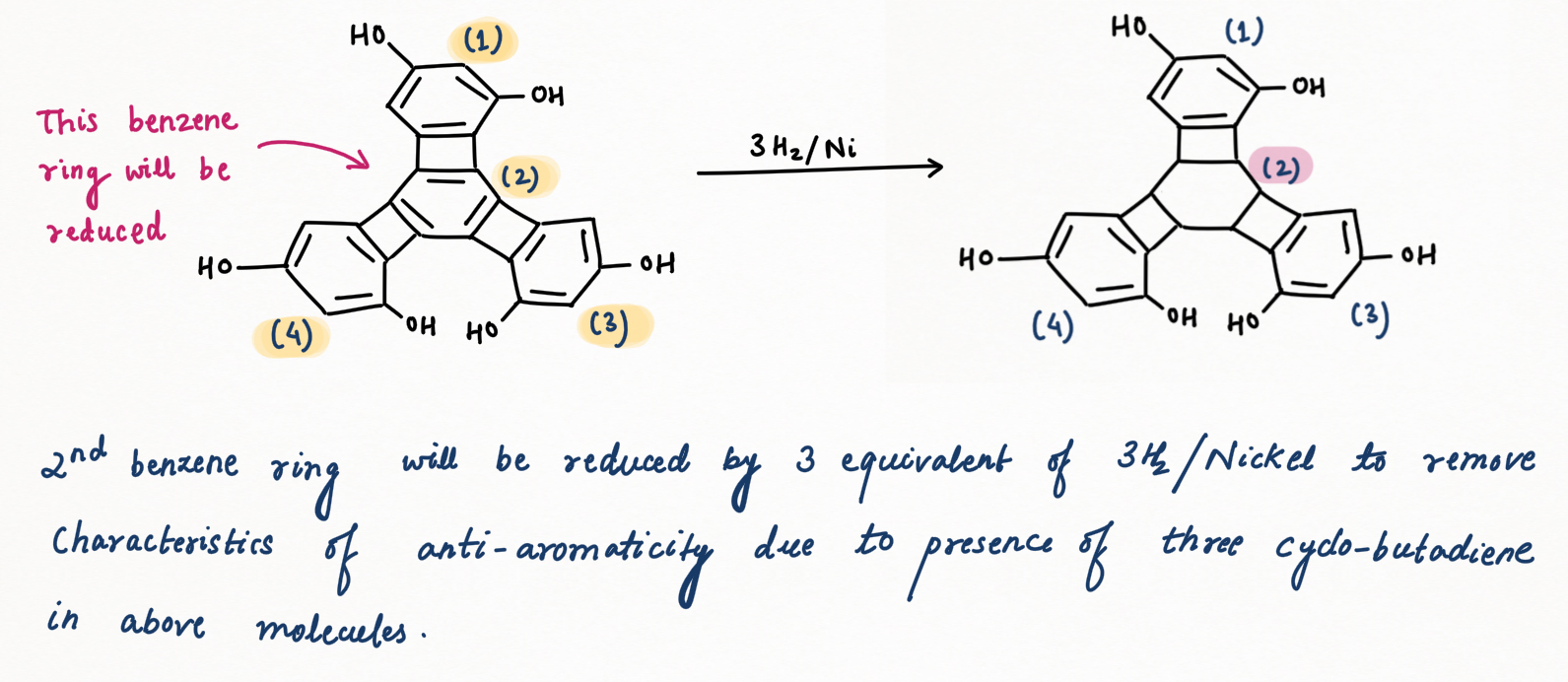
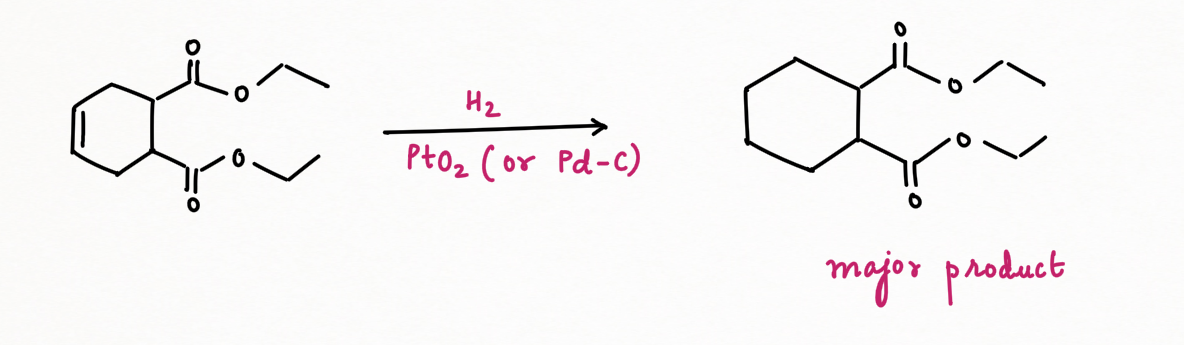Introduction
Catalytic hydrogenation is a tremendously important reaction. It is an
essential step in the synthesis of many fine chemicals as well as bulk
commodities. In catalytic hydrogenation, a pair of hydrogen atoms are added across a double bond, turning an alkene into an alkane.
Reason for using a catalyst in the hydrogenation of unsaturation:
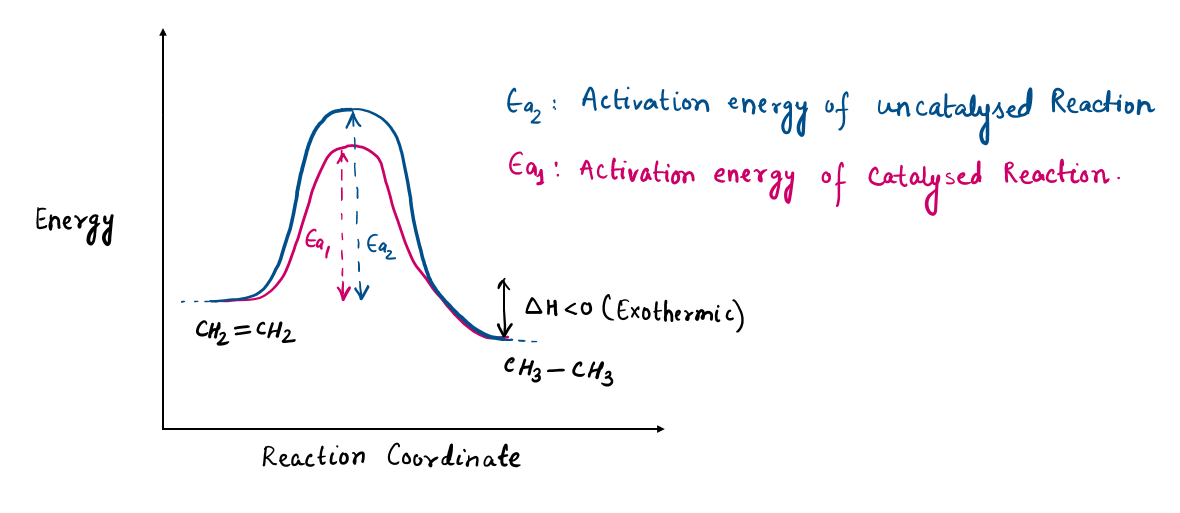
1. Time-Saving: the faster the catalyst, the more product can be made and the more economical the process, which means lowering activation energy to attain a reaction faster.
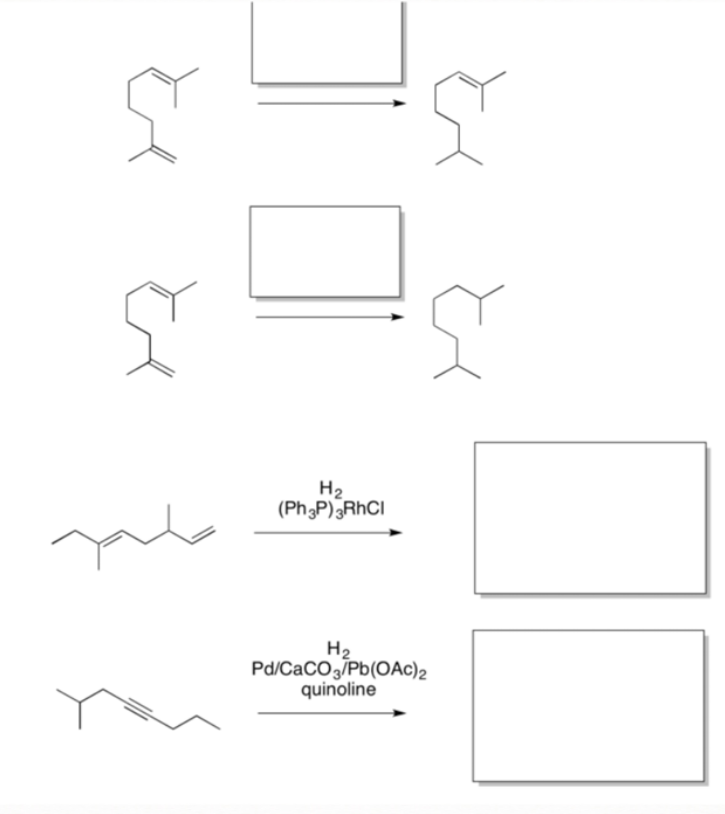
2. Selective Reduction: Suppose two double bonds are present
in a molecule. Maybe you only want to hydrogenate one of these double bonds. By choosing the proper catalyst, you may be able to do selective hydrogenation.
Selectivity
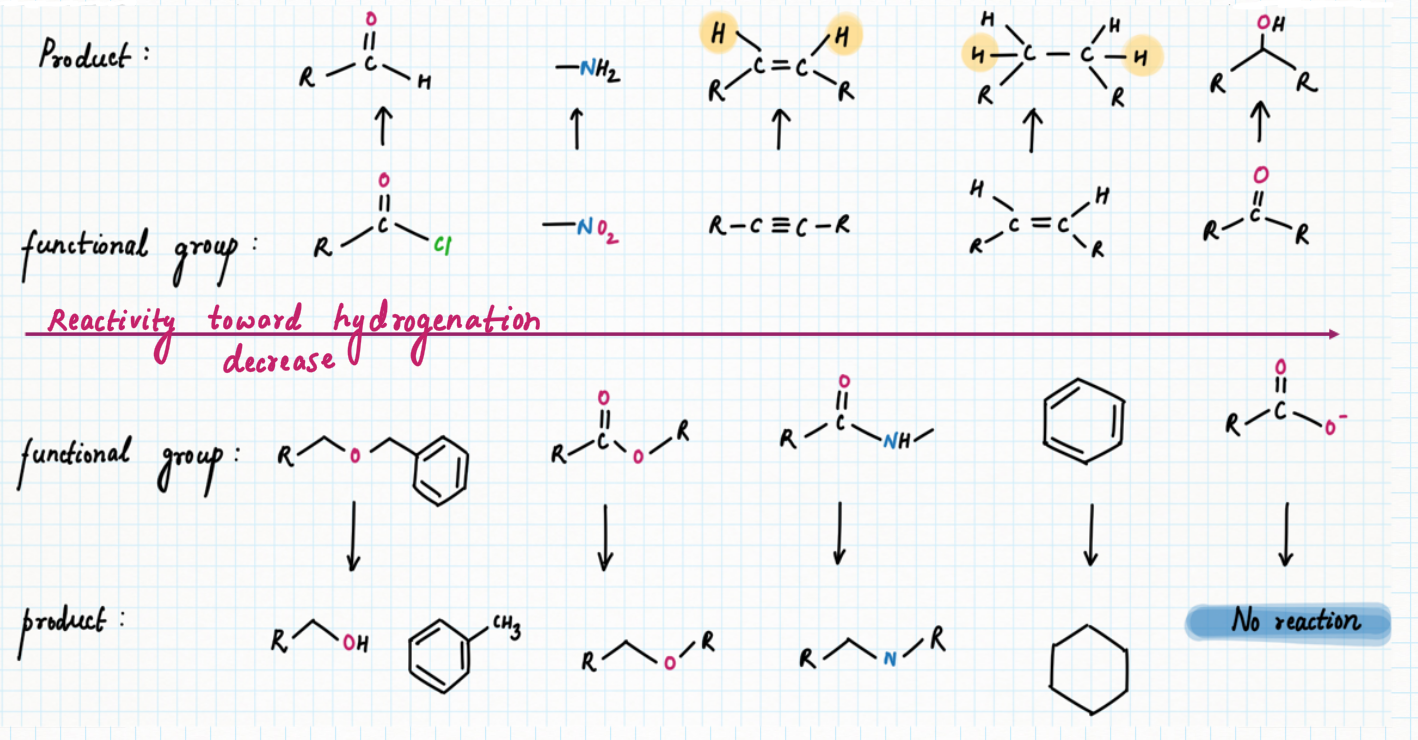
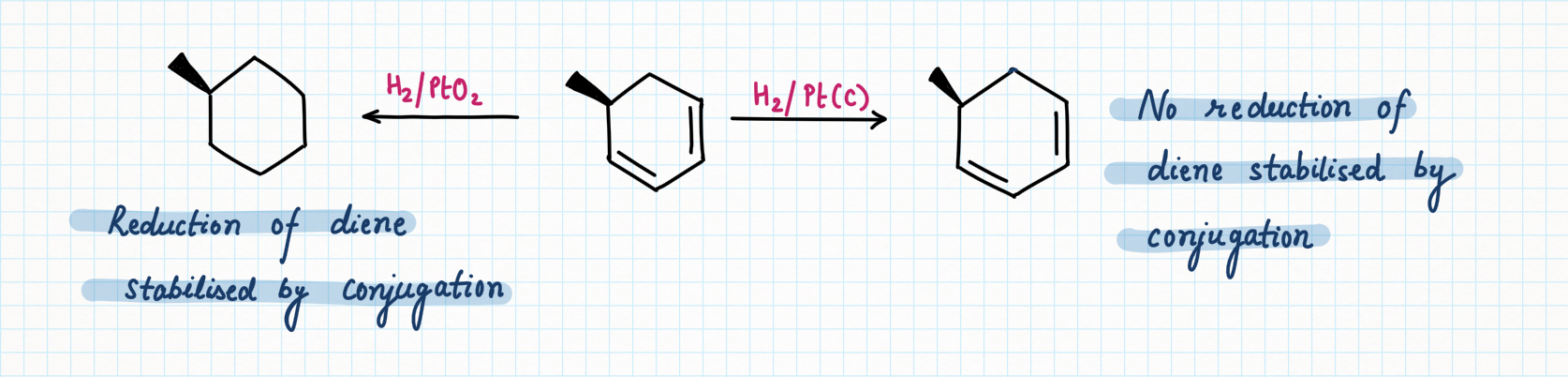
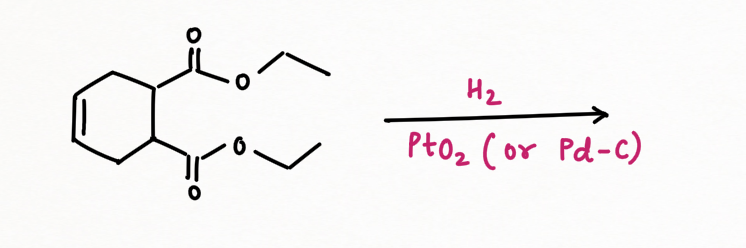
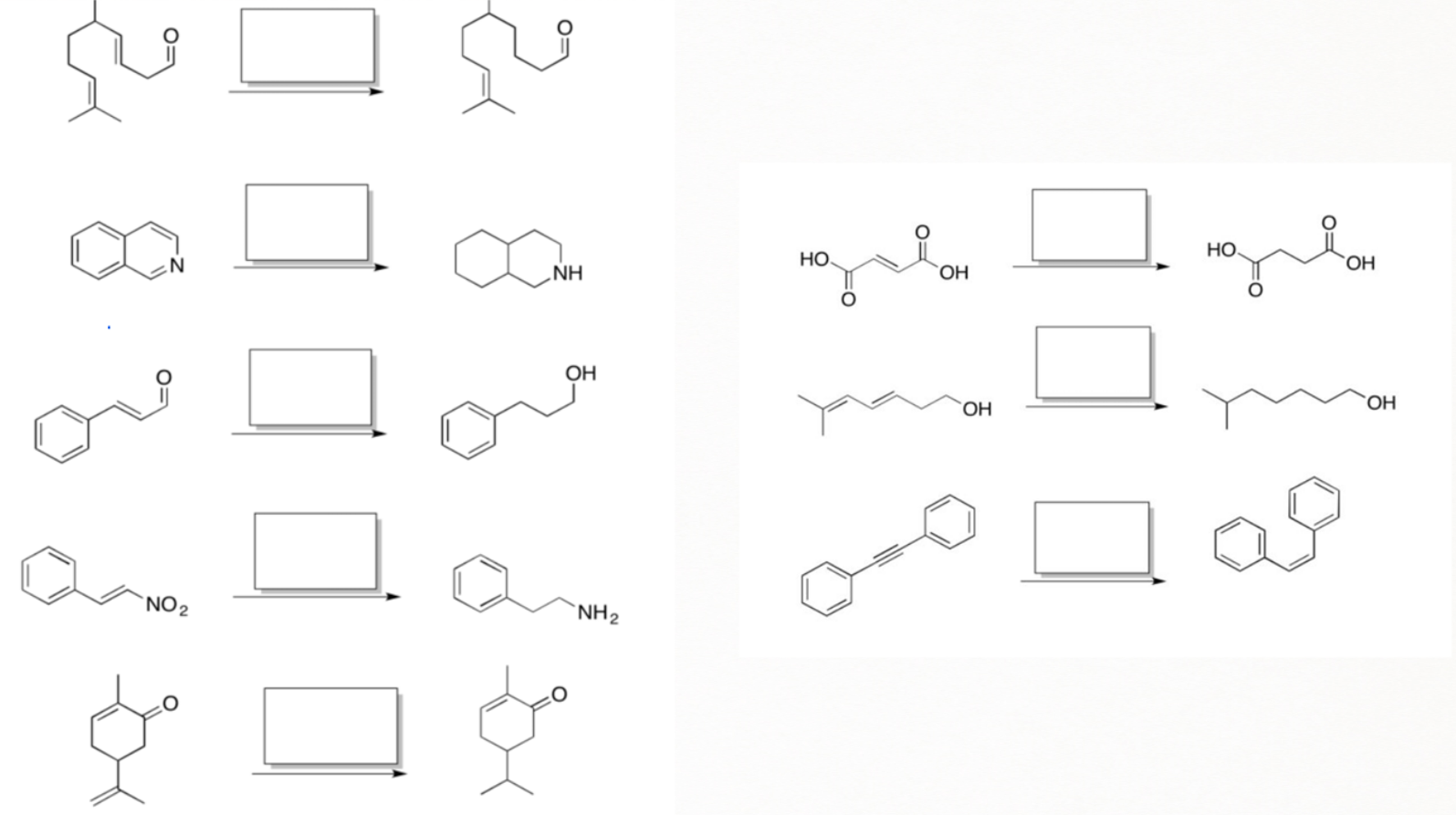
Palladium on carbon, or Pd/C (95% Carbon + 5 % palladium), is known for selective reduction or hydrogenation. It is very good at adding hydrogen to alkenes and alkynes, too. However, it is not very good at hydrogenating more stable double bonds, such as conjugated dienes, benzene, or other aromatics.
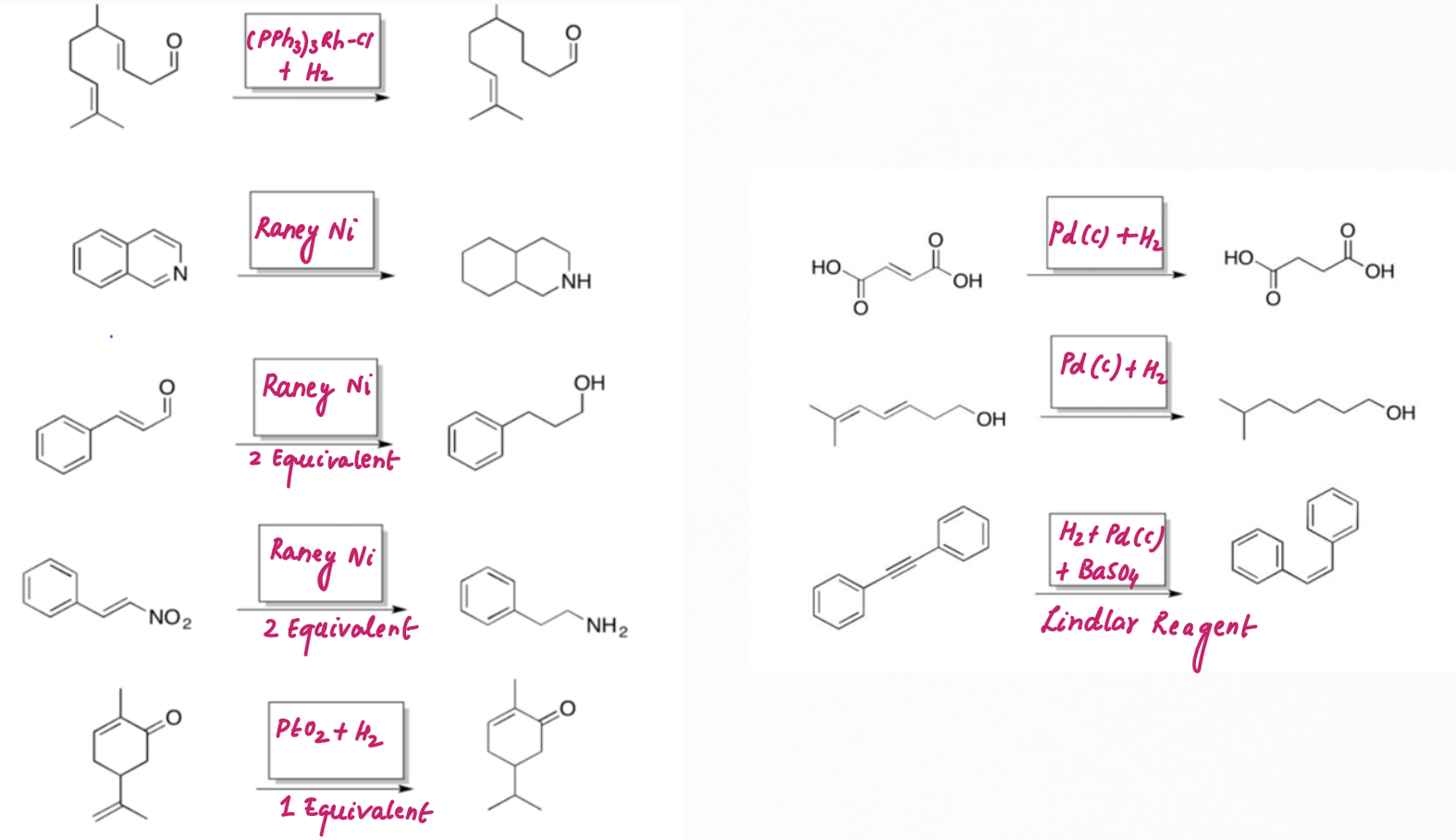
In contrast, platinum oxide (PtO2) is much more general, hydrogenating regular alkenes and conjugated ones as well as aromatic too.
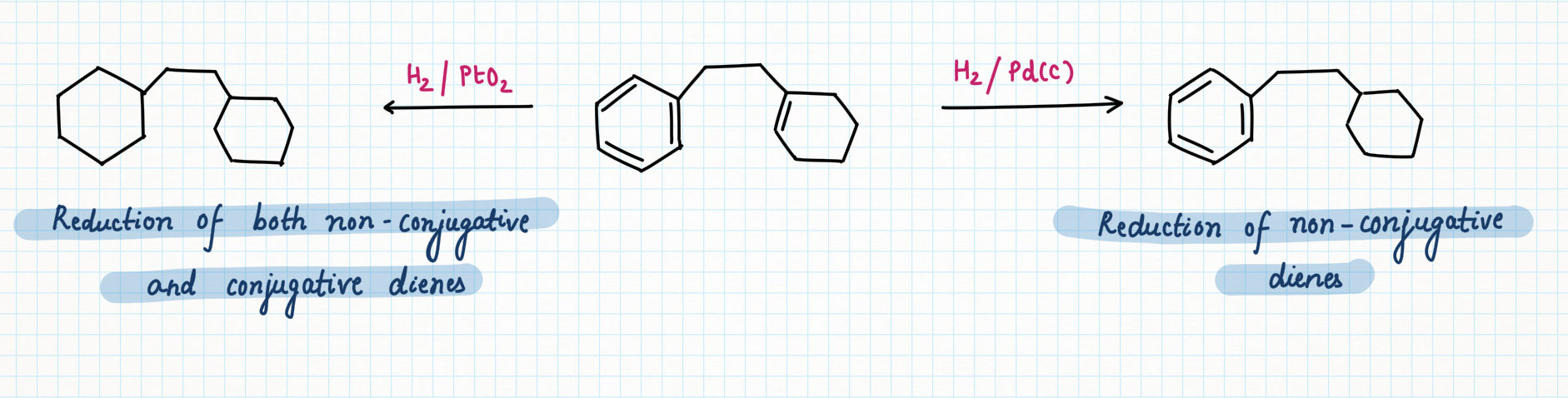
Versability
Reduction of carbonyl along with unsaturated bond :
However, hydrogenation with palladium or platinum oxide (PtO2) doesn't work well with carbonyls. It usually won't reduce aldehydes or ketones. But another heterogeneous catalyst, Raney Nickel (H2/ Ni) can reduce carbonyl groups such as aldehyde, and ketone but fail to reduce amides, esters, and carboxylic acids.
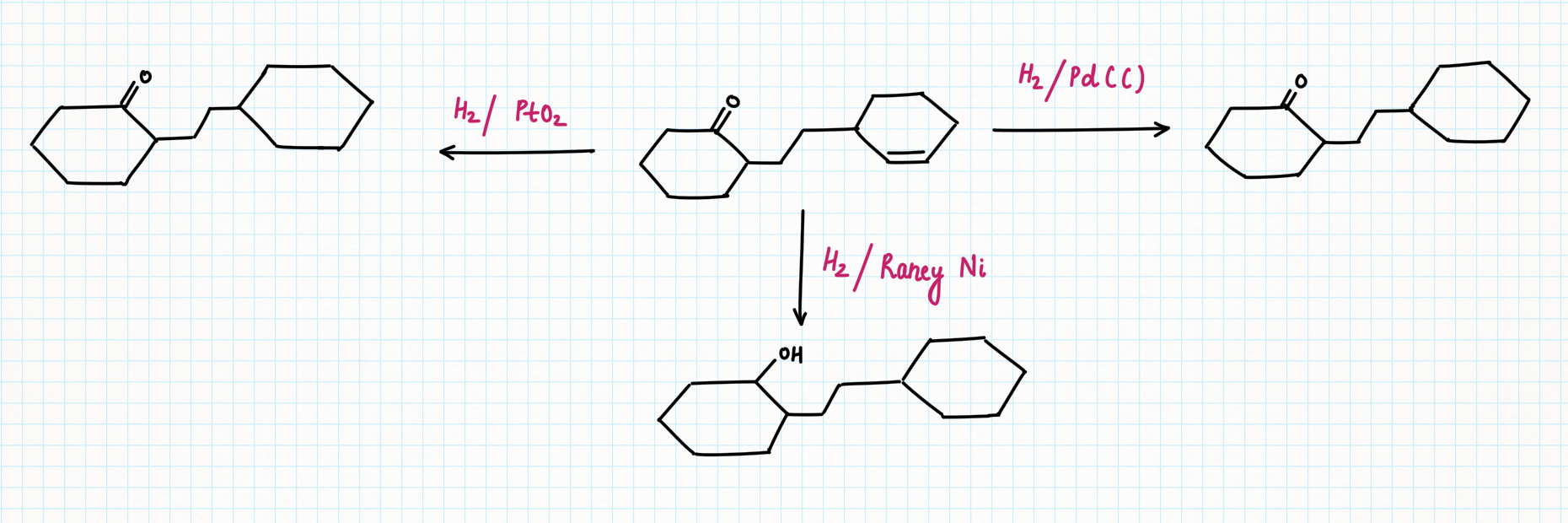
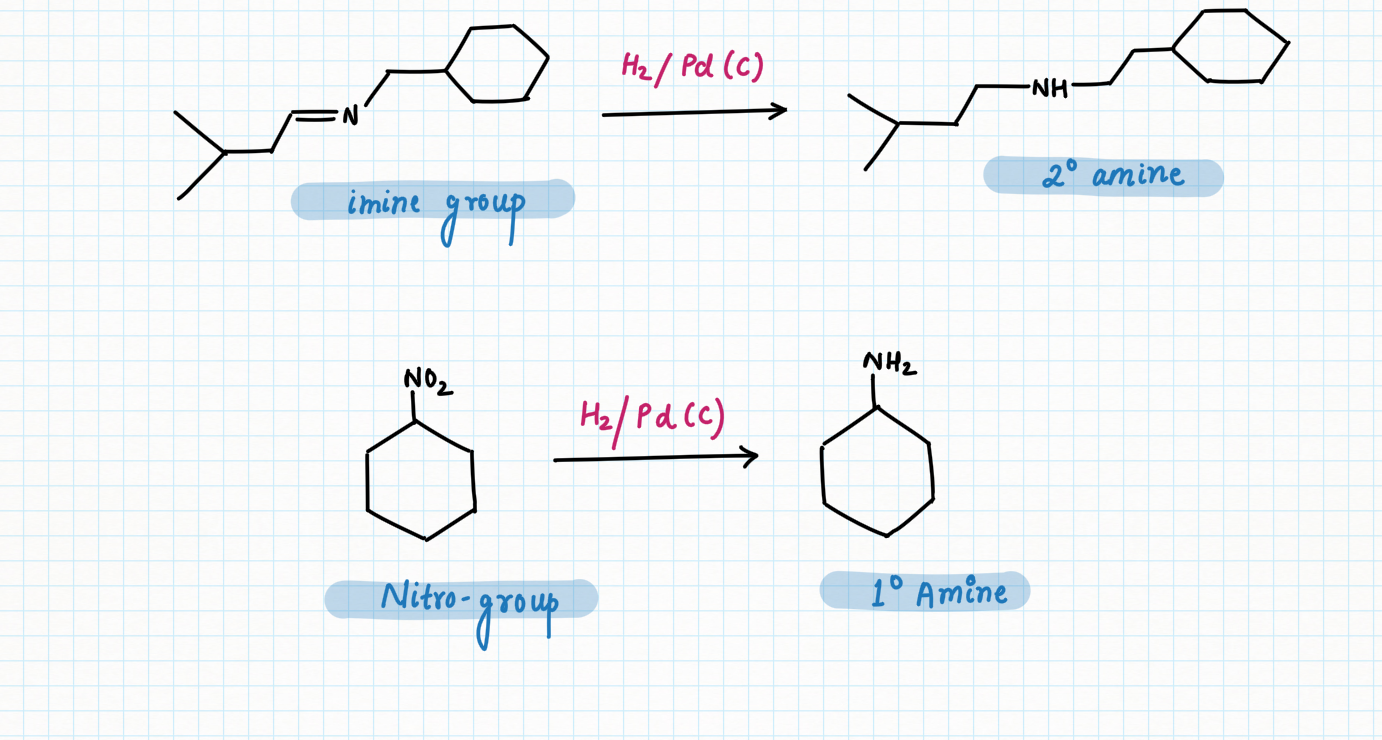
Reduction of N-containing functional group :
On the other hand, palladium or Raney Ni can reduce compounds,
containing imines and nitro groups, cyanide to their corresponding amines.
Poisoning of Surface for increasing selectivity
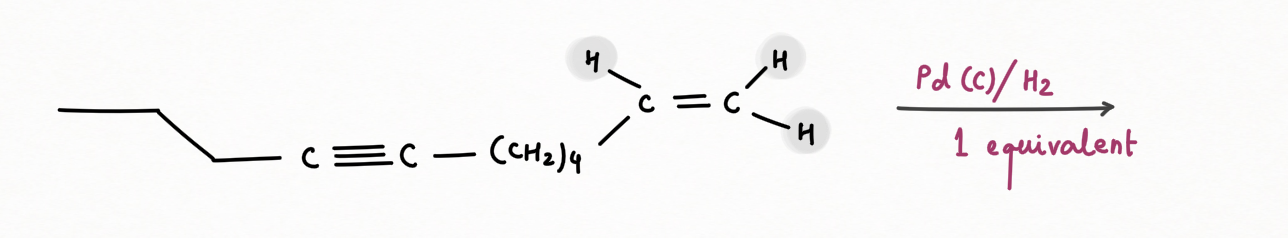
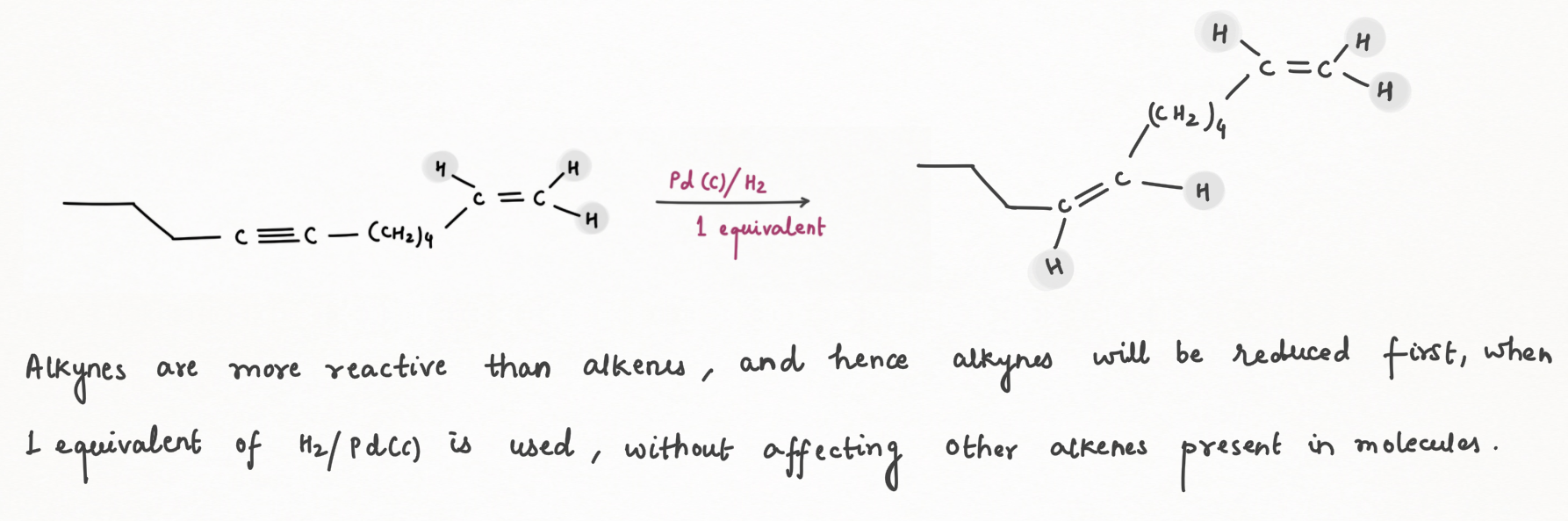
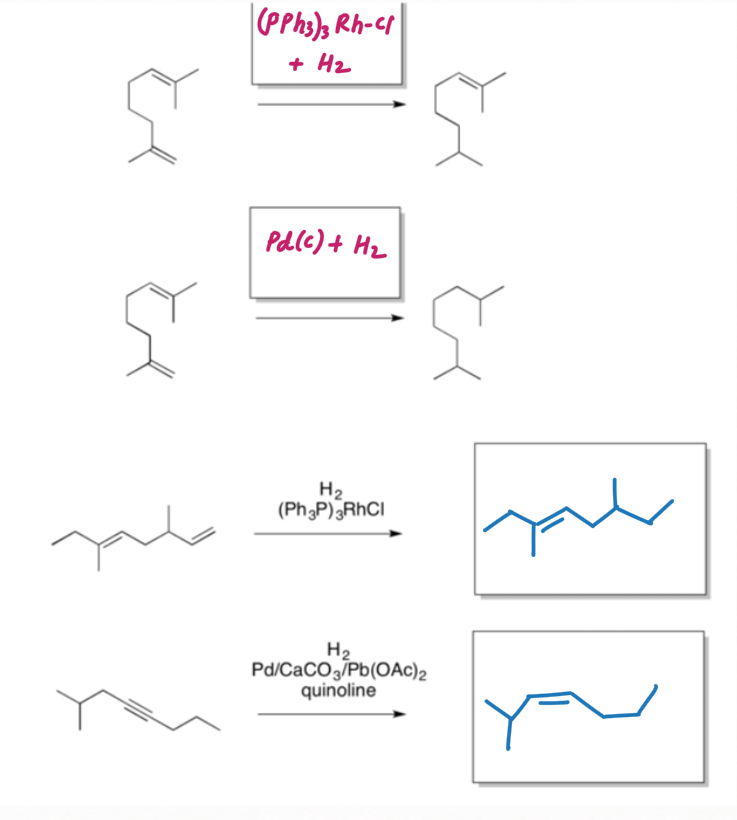
Reduction of Alkyne using Lindlar's Reagent :
We can make the palladium catalyst even more selective by preparing
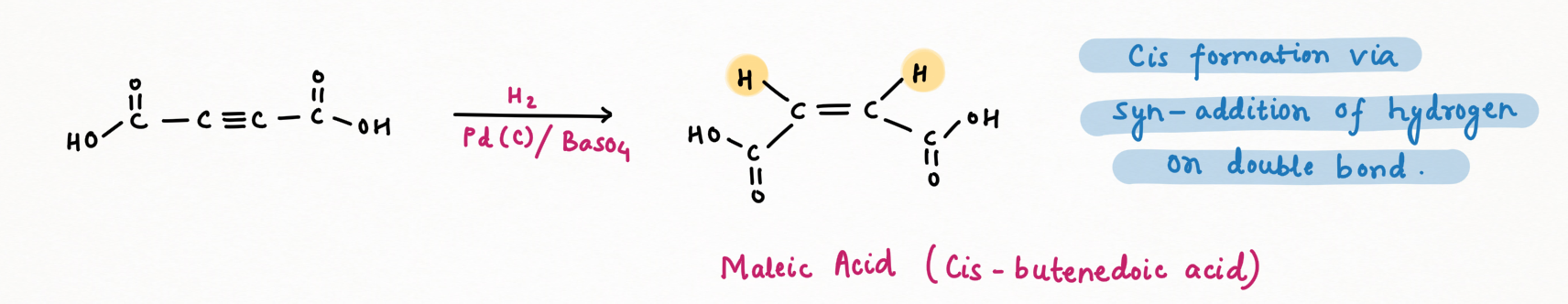
Lindlar's reagent. To make Lindlar's catalyst, palladium is supported on calcium carbonate (CaCO3) or barium sulfate (BaSO4) or carbon then poisoned with quinoline or lead acetate [ Pb (OCOCH3)2].
Lindlar's reagent is used for the hydrogenation of alkynes to alkenes without further reduction into alkanes.
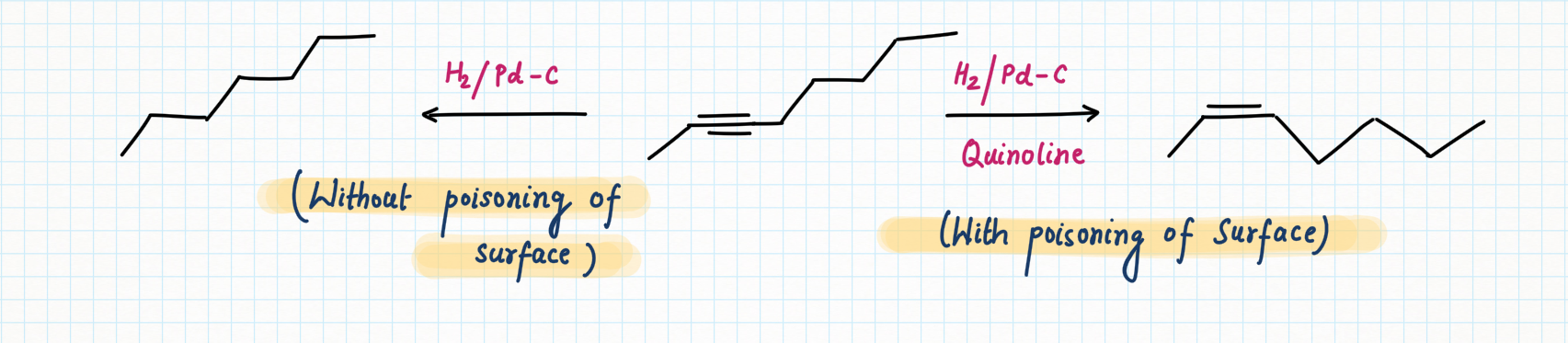
Alkyne hydrogenation by Lindlar's reagent is stereospecific occurring via syn addition giving cis-alkene
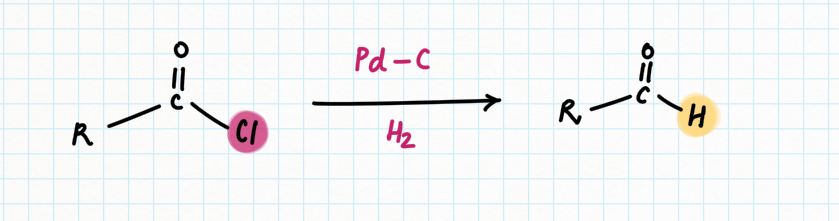
Reduction of Aldehyde: Rosenmund Reaction
When acyl-chloride is selectively reduced to an aldehyde, this reaction is known as the Rosenmund reaction.
Sensitivity to hindrance
Generally, fewer substitute alkenes are reduced before more substituted alkenes due to their lack of steric hindrance.

Let's understand the effect of a hindrance on hydrogenation through another illustration of bridgehead carbon having one double bond with methyl substituents on both sides, creating a significant crowd.
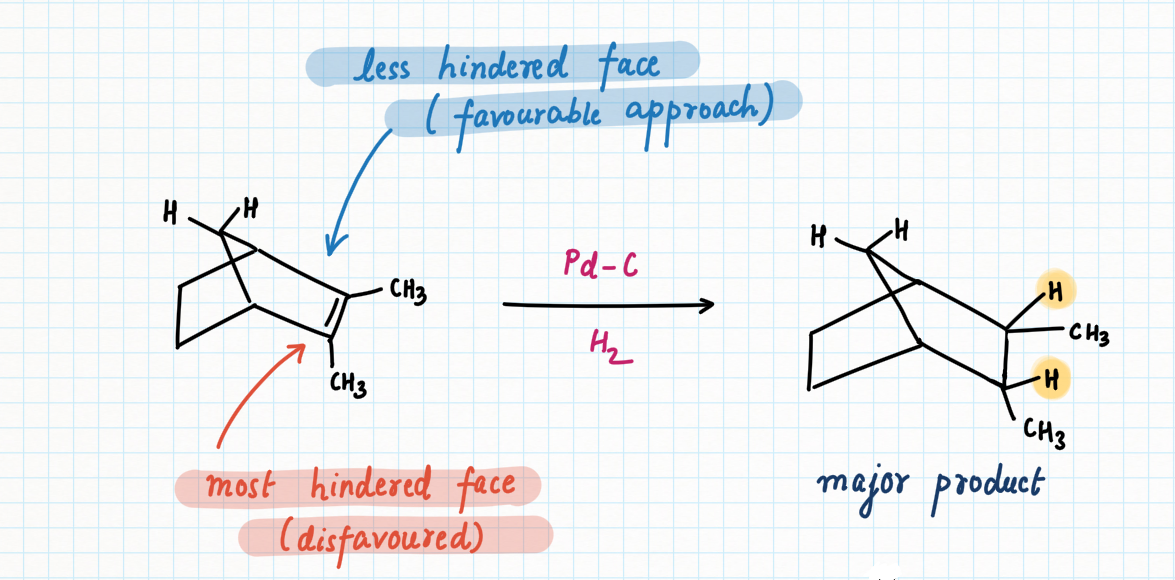
In this case, approach on the top face is favored because it is only blocked by a one-carbon bridge, whereas the approach on the bottom encounters a two-carbon bridge.
Wilkinson's catalyst (RhCl(PPh₃)₃) :
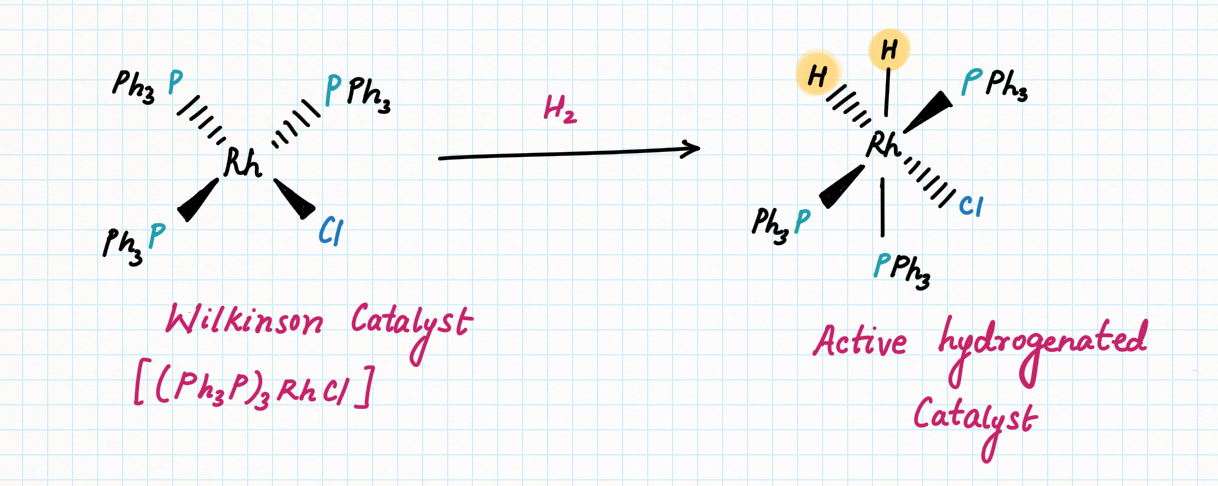
This catalyst is good at reacting with alkenes but leaving polar bonds alone. It is also highly selective, reacting only with the least sterically crowded alkenes. It only reacts with monosubstituted and disubstituted alkenes. If an alkene has groups other than two hydrogens attached to the double bond, Wilkinson's catalyst fails to reduce that bond.
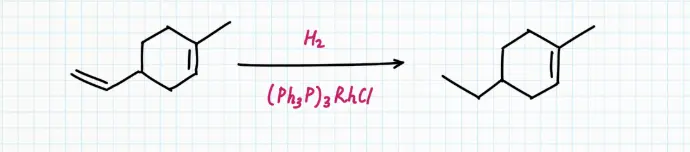
Variation of hydrogenation reactivity with pressure
As benzene rings are less reactive than alkenes, hence it is very difficult and not feasible to reduce benzene at normal conditions. So, for complete reduction, there is a need for high pressures to achieve complete hydrogenation of benzene.
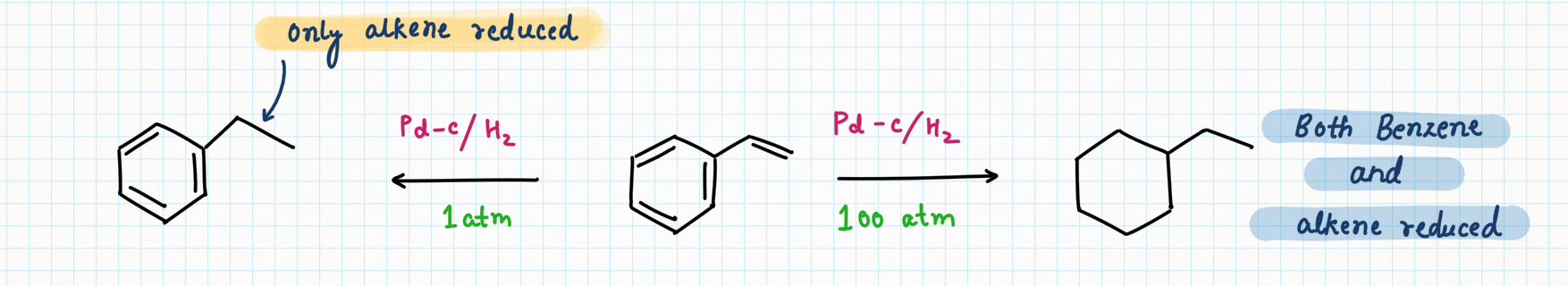
Raney nickel at 1 atm.
Stereochemistry of hydrogenation
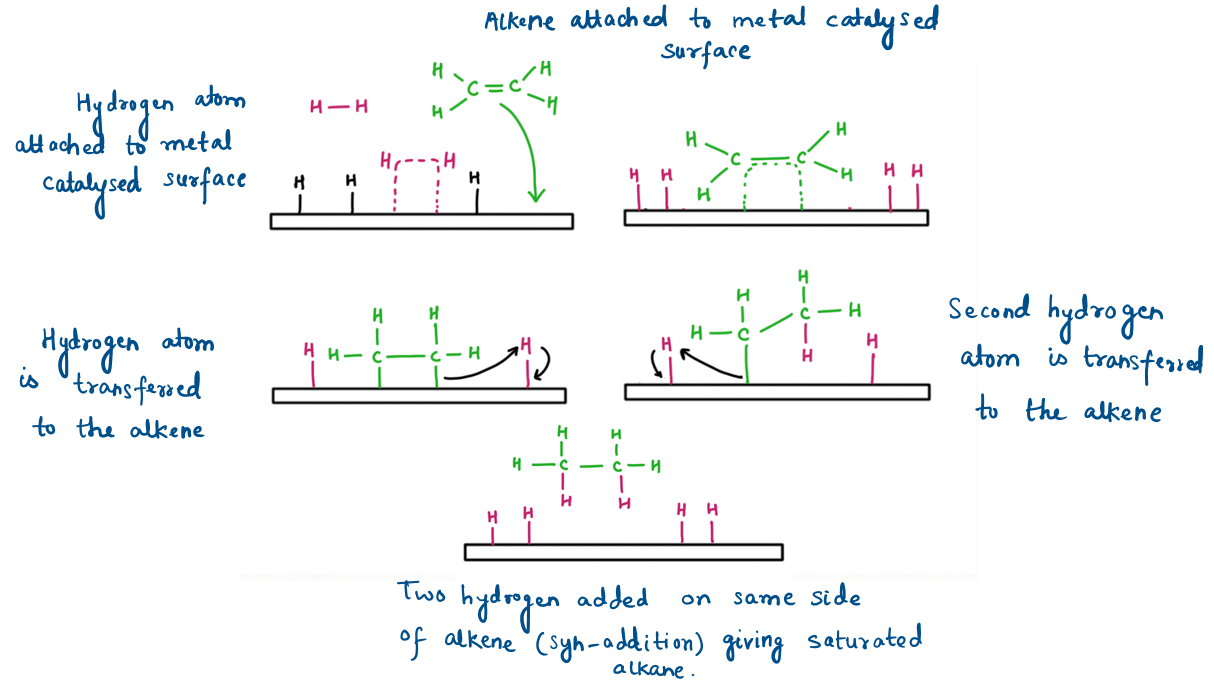
Hydrogenation is generally a stereoselective reaction occurring via syn-addition (addition on the same side of alkene) producing cis-product as a major product.
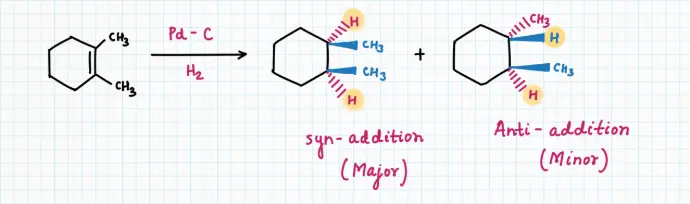
Sometimes the product can isomerize, leading to minor amounts of the anti-addition product — this can be minimized by using platinum (Pt) or with Wilkinson’s catalyst RhCl(PPh₃)₃.
Stereochemistry of hydrogenation using birch reagent :
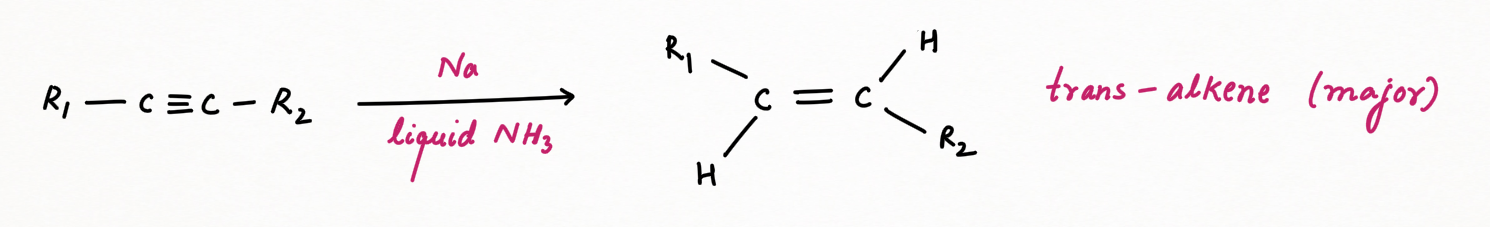
Non-terminal alkynes can reduced to trans-alkene as a major product, with sodium or lithium metal dissolved in liquid ammonia as a solvent. Unlike other reagents giving cis as a major, birch reagent gives trans-alkene as a major product.
Mechanism :
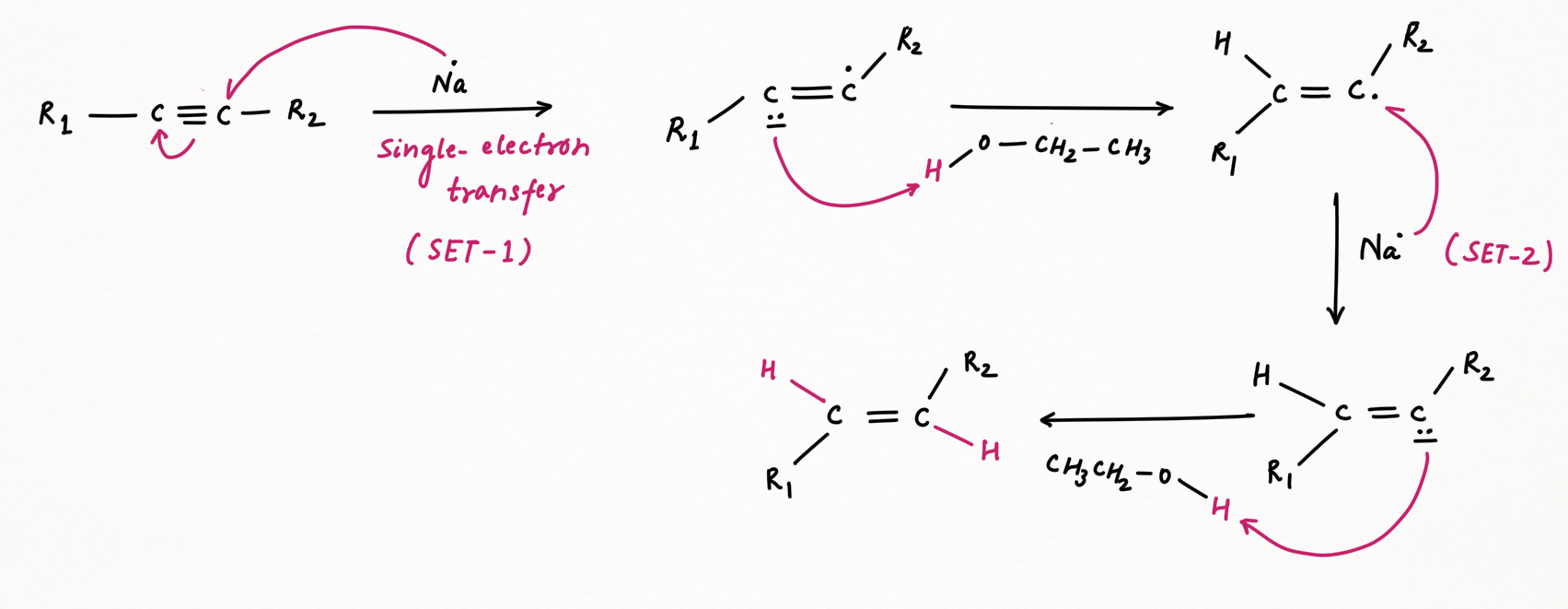
For terminal alkynes, acidic hydrogen of the end triple bond will perform an acid-base reaction with ammoniated sodium, instead of reduction of alkynes to trans-alkene.
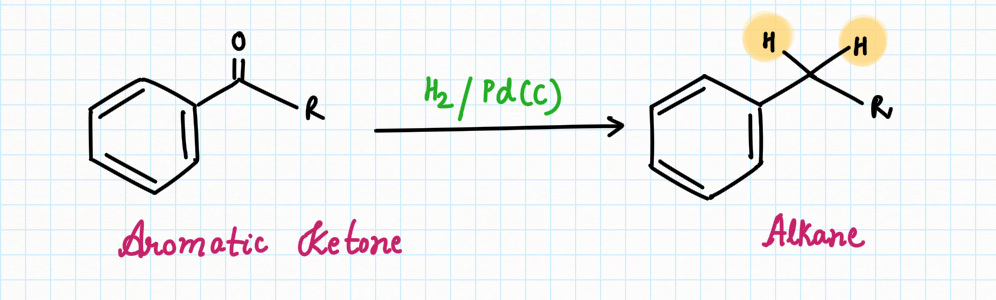
Reduction of aromatic ketone:
When treated with a metal catalyst such as palladium (Pd) or platinum (Pt) and hydrogen gas (H2), ketones next to aromatic groups are converted into alkanes :
Reduction of non-aromatic keto group :
Ketones of secondary non-aromatic can be reduced to alcohols using Pd/C with enough pressure of H2, but to go down to the alkane, it’s necessary to convert the OH into OTs (or OMs) and treat with LiAlH4.
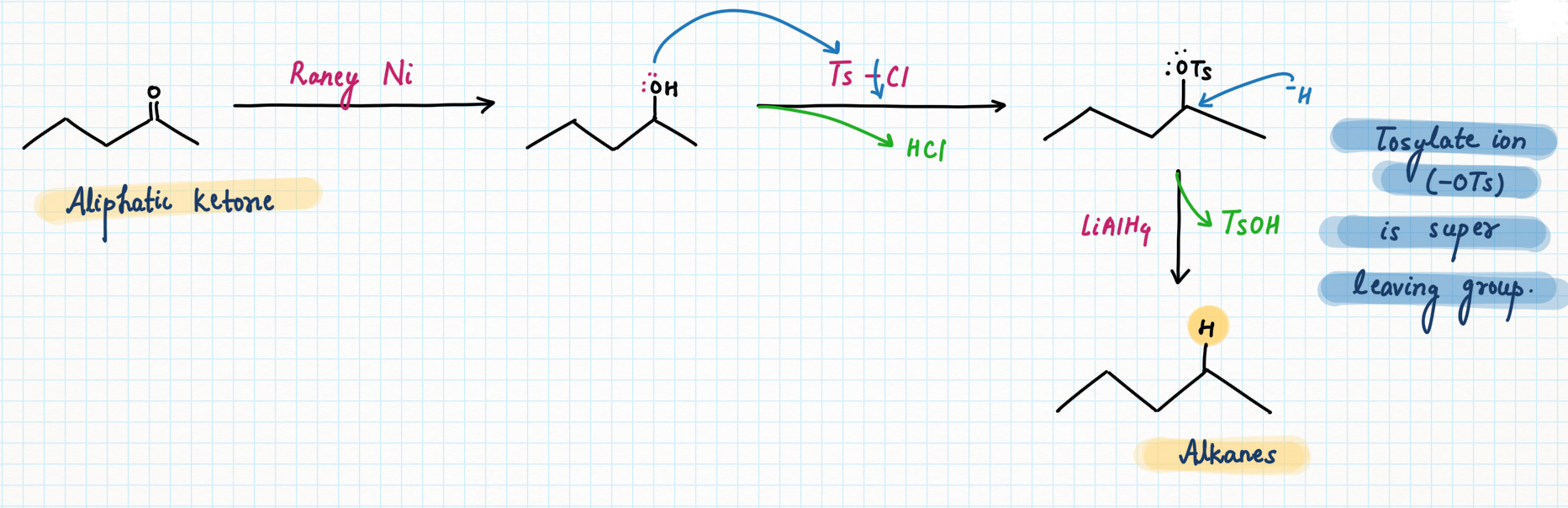
Essentially a substitution reaction occurs forming C-H and breaking C-O.
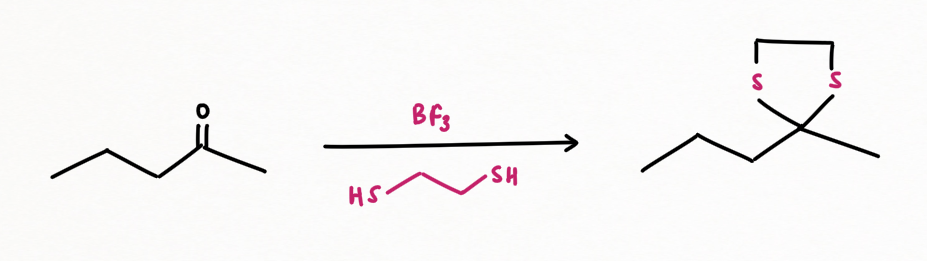
An alternative method for any keto-reduction: Desulphurization of thiol-acetal by Raney-Ni
Step 1: Thioacetal formation from keto
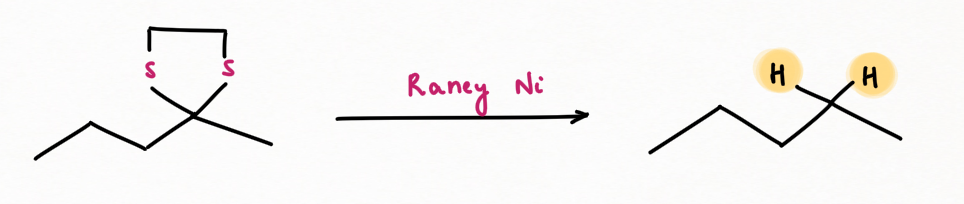
Step 2: Desulphurization of thioacetal by raney-Ni
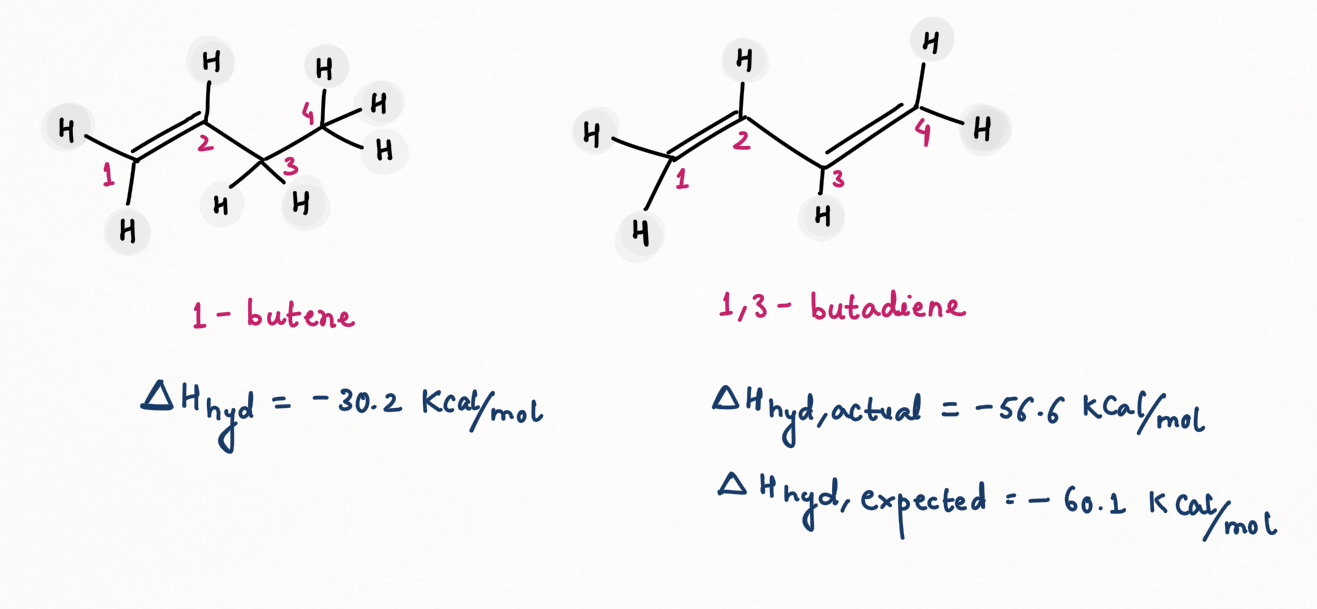
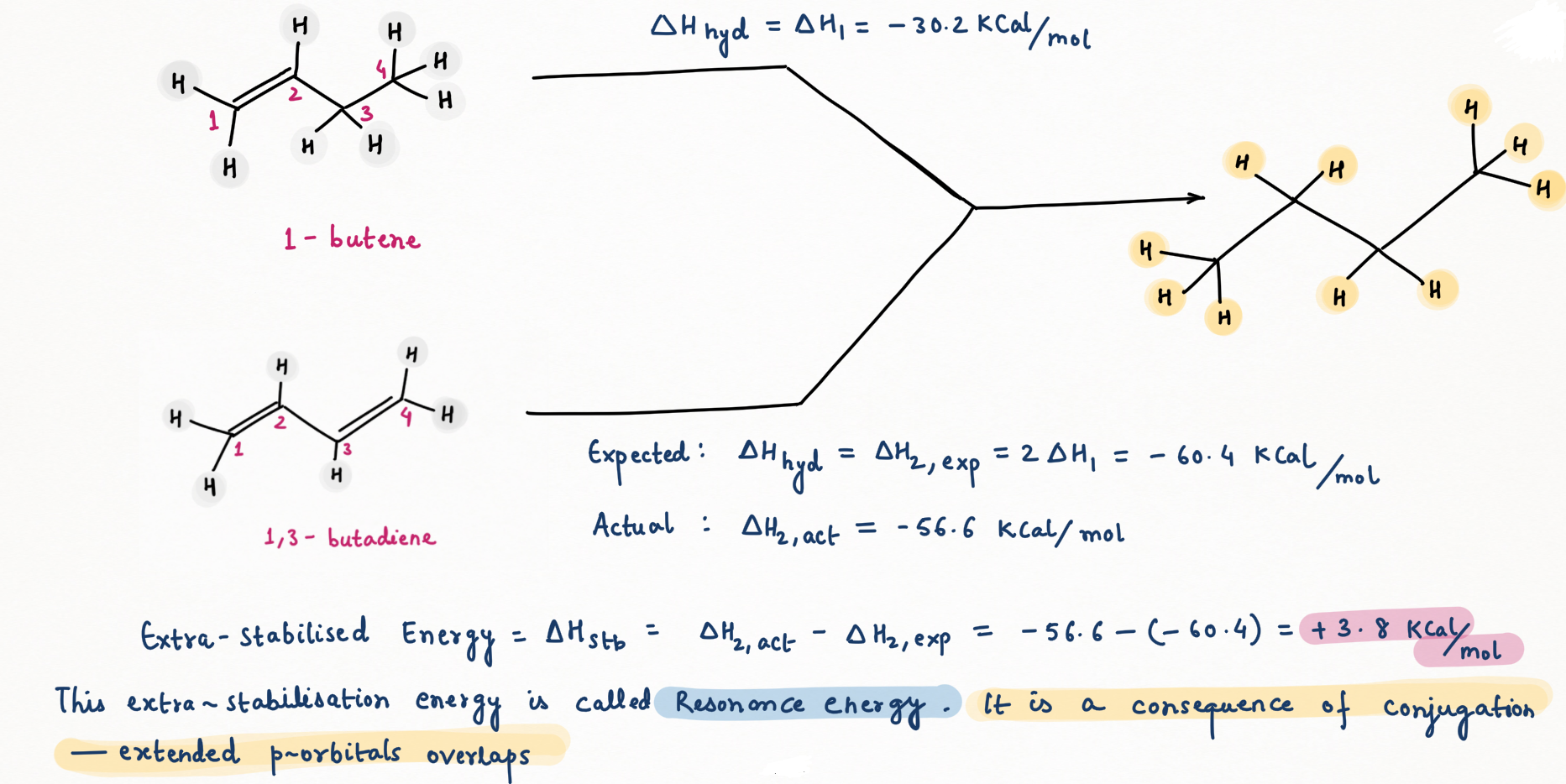
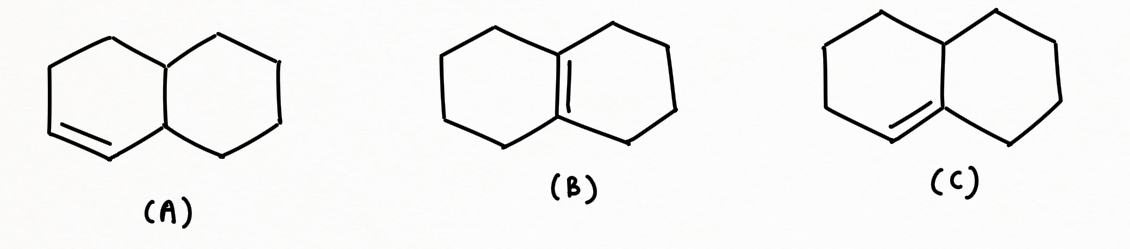
Heat of hydrogenation and stability of alkene
Hydrogenation of a double bond is a thermodynamically favorable reaction because it forms a more stable (lower energy) product. In other words, the energy of the product is lower than the energy of the reactant; thus it is exothermic (heat is released). The heat released is called the heat of hydrogenation, which is an indicator of a molecule’s stability.
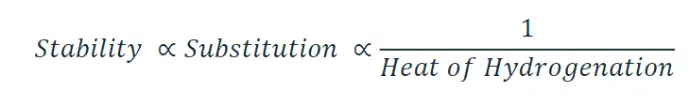
Some important points regarding for deciding the stability of alkene :
- Conjugation of alkene increases stability while strain decreases stability
- Stability of Di-substituted alkene: 𝑇𝑟𝑎𝑛𝑠 > 𝐶𝑖𝑠 ≈ 𝐺𝑒𝑚𝑖𝑛𝑎𝑙 (1,1)
- As ring size increases, the trans-cycloalkanes isomers become less unstable
Relative reactivity of different functional group