Understanding bond orders is akin to deciphering the language of molecules, revealing their stability and reactivity. In this exploration, we embark on a journey to demystify the calculation of bond orders for both diatomic and polyatomic molecules, unraveling their intricacies one step at a time.
A. Diatomic Molecules:
- Diatomic Molecules with N ≤ 8:
- If the total electron count (N) is odd, the bond order is set at 0.5.
- If N is an even number and not a multiple of 4, the bond order stands at 1.
- If N is even and a multiple of 4, the bond order drops to 0.
- Be2, with N = 8, exhibits a bond order of 0, rendering its existence improbable.
- Li2, with N = 6, showcases a bond order of 1, highlighting its potential for stability.
- Diatomic Molecules with N > 8:
- Bond Order = 3 - |(14 - N)/2| (Ensuring positivity)
- N2+, with N = 13, demonstrates a bond order of 2.5, signifying moderate stability.
- O2-, boasting N = 17, presents a bond order of 1.5, indicative of its resilient nature.
B. Polyatomic Molecules:
- Begin with the construction of the Lewis structure.
- Tally the total number of bonds within the molecule.
- Identify the count of bond groups between individual atoms.
- Calculate the bond order by dividing the number of bonds by the total bond groups.
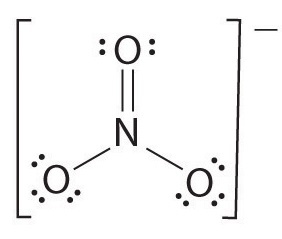
Example: NO3-
- With 4 bonds and 3 atoms surrounding the central nitrogen, the bond order is computed as 4/3, yielding 1.33.
Example: Ozone (O3)
- Featuring 3 bonds and 2 atoms adjacent to the central oxygen, the bond order equates to 3/2, showcasing a value of 1.5.
With each calculation, we unravel the intricate dance of electrons within molecular realms, offering glimpses into their inherent stability and behavior. Embrace the beauty of chemistry as we decode the language of molecules, one bond order at a time.
Abnormal Bond Order of CO+ with a Logical Explanation :
Join us in this voyage of discovery and mastery, where every calculation illuminates the path to deeper comprehension and appreciation of the molecular world.
Don't forget to engage with us on this journey by sharing your insights and experiences. Together, let's delve deeper into the captivating realm of chemistry.
Keep exploring, keep learning, and let the bonds of knowledge propel you to new heights in your scientific endeavors! 🧪✨