Hey there, future chemists!
Let's dive into the fascinating world of Lithium Borohydride (LiBH₄), an incredible reducing agent with some unique properties that are sure to catch your interest and might just trend in the coming years. Here’s why LiBH₄ deserves your attention:
What Makes LiBH₄ Special?
- Selective Reduction of Esters:
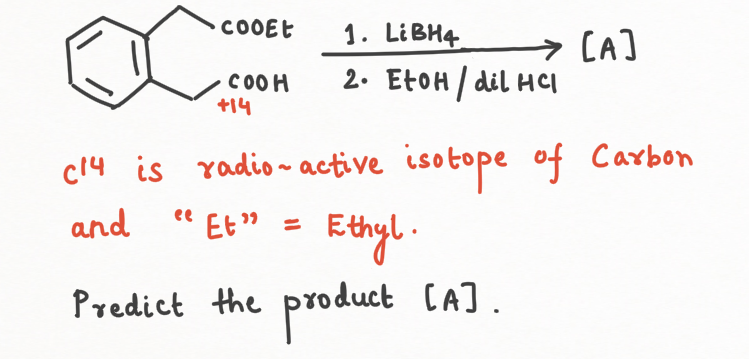
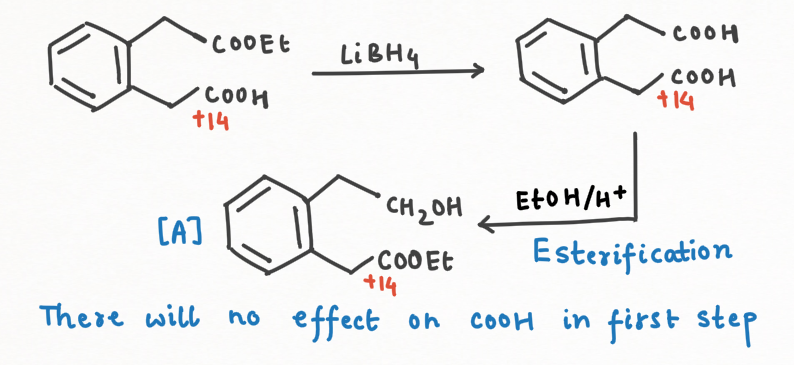
- Reduction to Alcohols: LiBH₄ is exceptional at reducing esters to alcohols, but it won't touch carboxylic acids. This selective reactivity makes it a powerful tool in organic synthesis, especially in multi-functionalized molecules where selective reduction is crucial.
- Strength of Reduction:
- Hydride Power: LiBH₄ is a stronger hydride reducing agent than NaBH₄ but not as potent as LiAlH₄. It strikes a perfect balance, making it suitable for specific reactions where NaBH₄ falls short and LiAlH₄ might be too aggressive. Its moderate reactivity allows for smoother and more controlled reductions.
- Versatile Applications:
- Reduction of Nitriles and Amides: This mighty reagent can reduce nitriles and primary amides to amines, opening up possibilities for synthesizing a wide range of amine compounds.
- Epoxide Opening: LiBH₄ can also open up epoxides, which is valuable in forming alcohols from epoxides. This reaction is particularly useful in ring-opening polymerization and organic synthesis.
- Selective Non-Reduction: Interestingly, it leaves nitro groups, alkyl halides, and tertiary/secondary amides untouched, providing precision in multi-step synthesis where selective reduction is needed.
- Reduction of Carboxylic Acid Derivatives:
- LiBH₄ can reduce carboxylic acid derivatives such as acyl chlorides and esters to primary alcohols. This makes it highly useful in the conversion of more reactive derivatives without over-reducing the substrate.
- Compatibility with Various Solvents:
- LiBH₄ is soluble in a variety of solvents including ethers and THF (tetrahydrofuran), which broadens its applicability in different reaction environments. Its compatibility with these solvents allows for greater flexibility in designing reaction conditions.
- Mechanism and Conditions:
- Reaction Conditions: LiBH₄ often operates under milder conditions compared to LiAlH₄, making it safer and easier to handle in a laboratory setting.
- Mechanistic Insights: Understanding the mechanistic pathways of LiBH₄ can aid in predicting its behavior in complex reactions, allowing for more strategic planning in synthetic chemistry.
Combustion of Lithium Borohydride, followed by reaction with liquid oxygen
Applications in Industry:
- Pharmaceuticals: LiBH₄ is employed in the synthesis of pharmaceuticals, where selective reduction is crucial for the production of specific drug molecules.
- Organic Synthesis: Its ability to selectively reduce and avoid over-reduction is beneficial in creating complex organic molecules with high precision.
As you delve deeper into chemistry, understanding the behavior of reagents like LiBH₄ can give you an edge in both academic and practical applications. Keep an eye on this topic—it’s set to trend in 2024 and beyond!
Question Practice