Introduction
How it looks like actually:
White solid with a strange, pungent smell. It looks candy ball.
Interestingly, it is often described as having ‘an odor of mothballs’
IUPAC Naming:
Bicyclo[4.4.0]deca-1,3,5,7,9-pentene.
Nobody is going to expect you to cram or recall such a long name. It's better to call naphthalene. Simple 😉
Degree of Unsaturation (DU) or Double Bond Equivalent (DBE)
D.U. of Naphthalene =
No. of ring + No. of pi-bond = 5 + 2 = 7
In another way, we can evaluate D.U through general mathematical equations,

Whereas,
Ni = No. of atom of each element
Vi= Valency of that element
Hence, for DU of naphthalene ( C10H8),
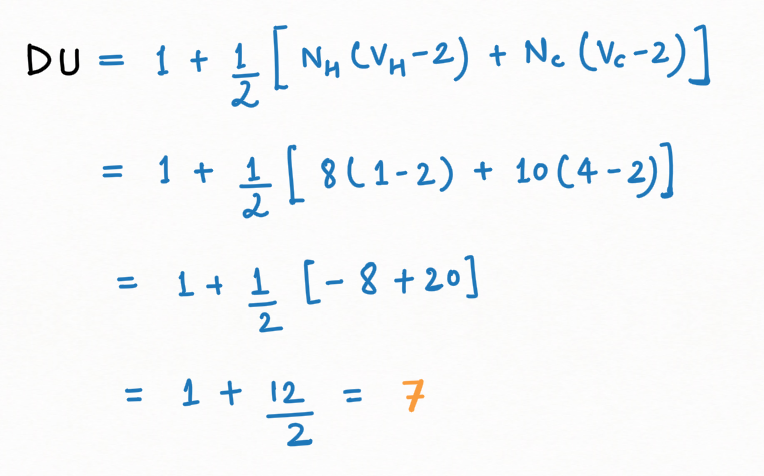
If you don't know how to calculate D.U.for 2-D or 3-D structure, then you can refer to our post where I have discussed 4-5 methods to evaluate DU (Click here).
Discovery:
Naphthalene was first reported in 1819 by Alexander Garden, a Scottish chemist. Then, Michael Faraday identified its molecular formula as C10H8 in 1826, and finally had its structure discovered in the 1860s
Alexander Garden
It was first reported by Alexander Garden, a Scottish chemist who ran a chemical supply shop in London
Michael Faraday
identified its molecular formula as C10H8
Finally, its structure was discovered completely.
Postmortem of Structure
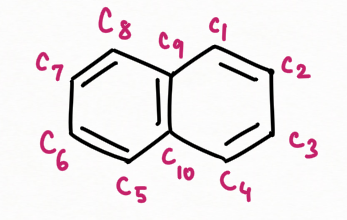
Correct Numbering on Carbon in Naphthalene :
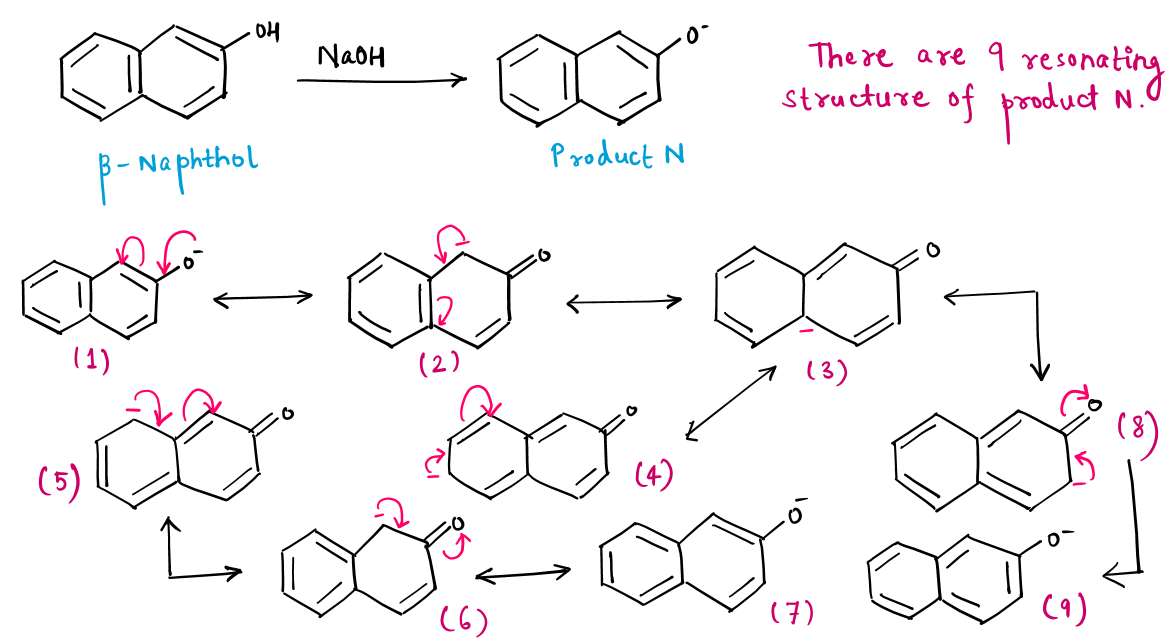
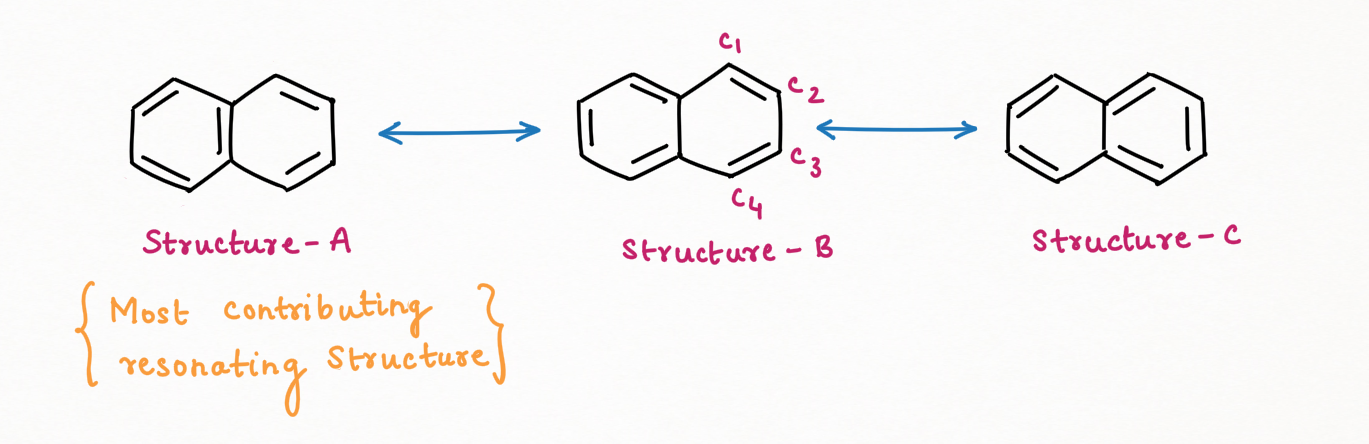
Resonating Structure :
There are 3 resonating structures of Naphthalene
Comment on the relative comparison of bond length :
The bond, C1-C2 has a double bond in Structure A and B while C2-C3 has a double bond in Structure C only.
Hence, bond length: C2-C3 (142 nm) > C1-C2 (135 nm)
C9-C10 has the shortest bond length in Naphthalene (133 nm) due to two reasons:
a) Cross Conjugation.
b) Rehybridization of orbitals junction under steric hindrance.
While C9-C1 is considered to have the longest bond length (142.2 nm).
Electrophilic Substitution Reaction:
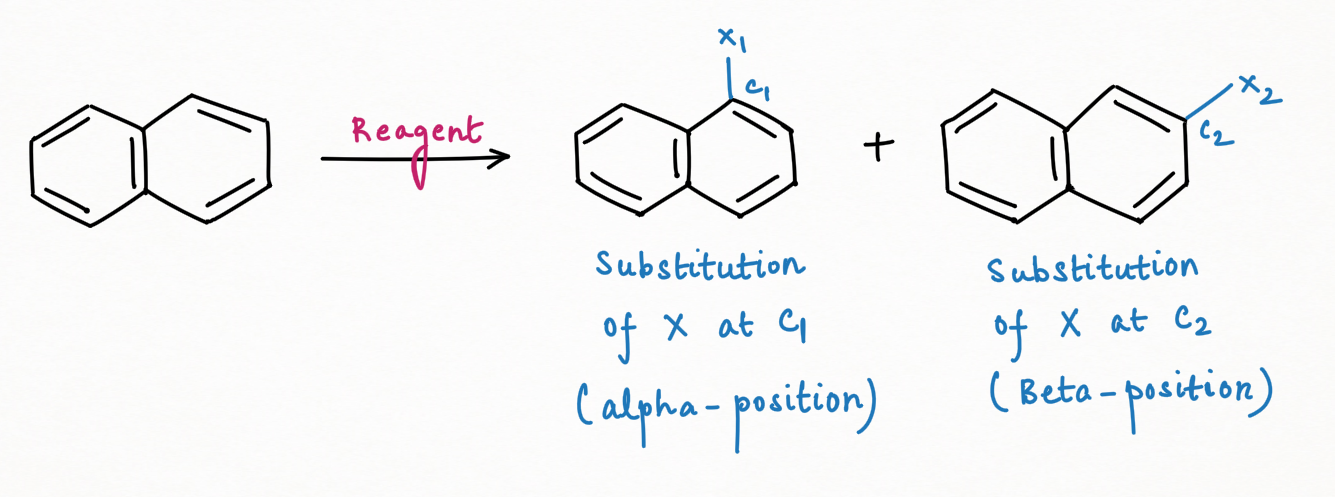
For X1-substitution at C1 carbon atom:
Nature of Substituents (X1) | Number of resonating structure | Number of aromatic resonating structure |
Electron Withdrawing Group | 7 | 4 |
Electron Releasing Group | 9 | 9 |
For X2-substitution at C2 carbon atom:
Nature of Substituents (X2) | Number of resonating structure | Number of aromatic resonating structure |
Electron Withdrawing Group | 6 | 3 |
Electron Releasing Group | 9 | 9 |
Nitration:
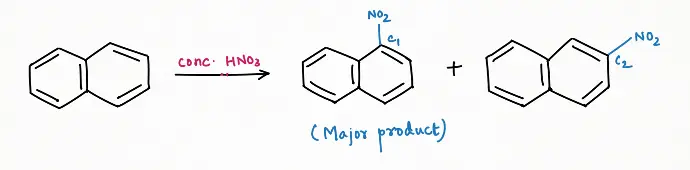
Generally, nitro-substitution is favored at C1 because of the more resonating structure of the product side ( C10H8-X1)
a) At C1, C10H7-X1 has 7 resonating structures, among which 4 have properties of aromaticity
b) While at C2, C10H7-X2 has a total of 6 resonating structures, out of them 2 structures have properties of aromaticity
Sulphonation:
Sulphonation of naphthalene is a highly reversible reaction.
Depending on the temperature, sulphonation of naphthalene gives two products such as:
a) Kinetic products, dominate at lower temperatures and depend on how fast the product is formed.
b) Thermodynamic product, at higher temperature and depending on the stability of the product.
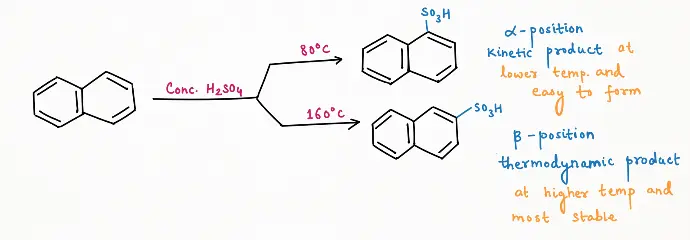
We have already discussed the concept of thermodynamic and kinetic products deeply in the brain teaser question blog. (Click Here)
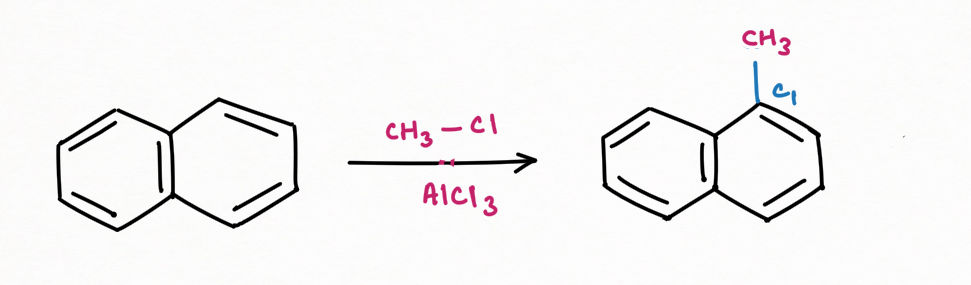
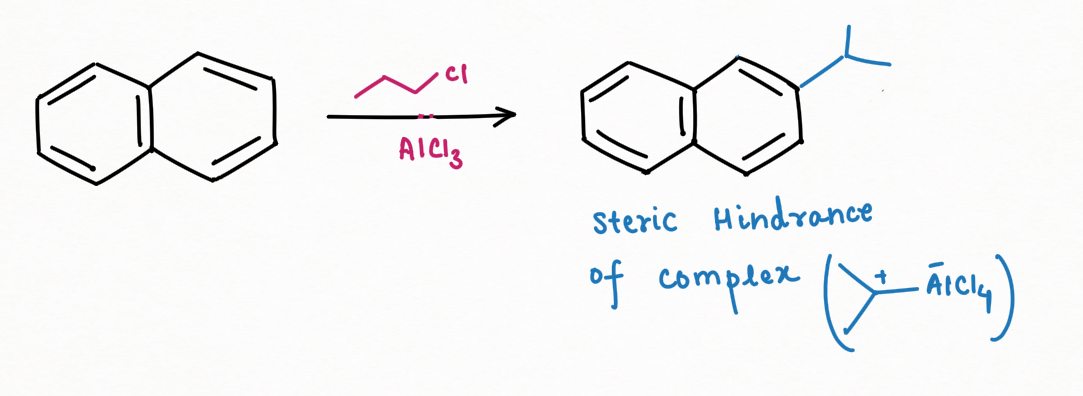
Friedel-Craft Alkylation:
The substitution of alkyl to naphthalene is generally favorable at C1, carbon atom. If the substituent is too large enough to create steric hindrance, then the substitution will occur at C2, carbon atom.
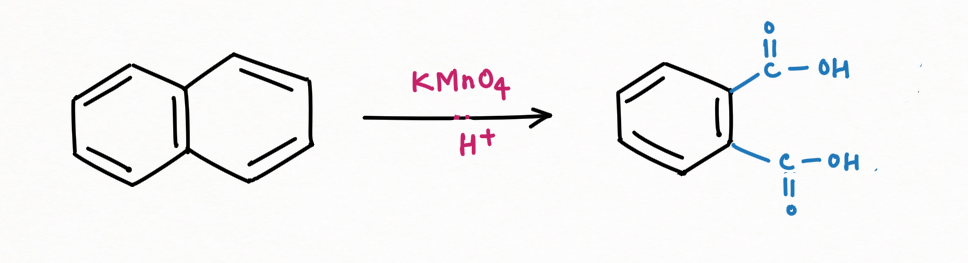
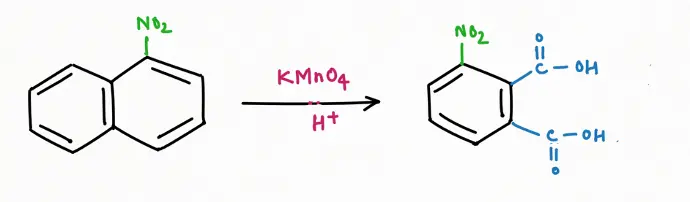
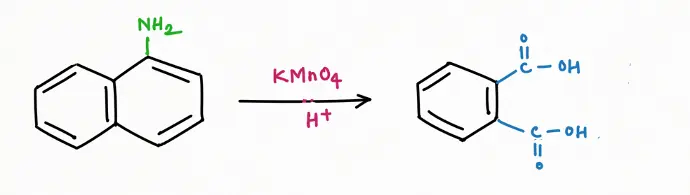
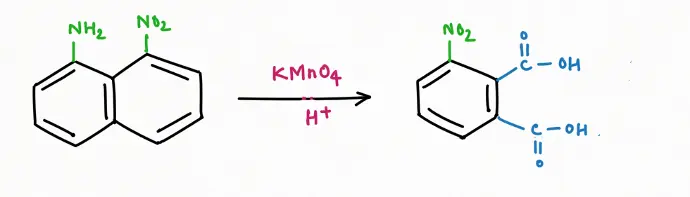
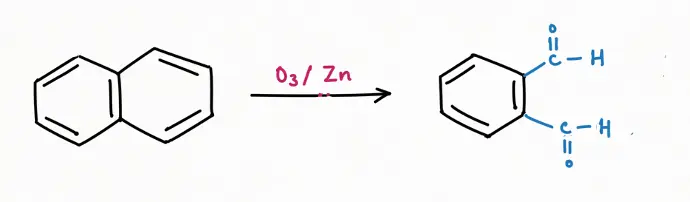
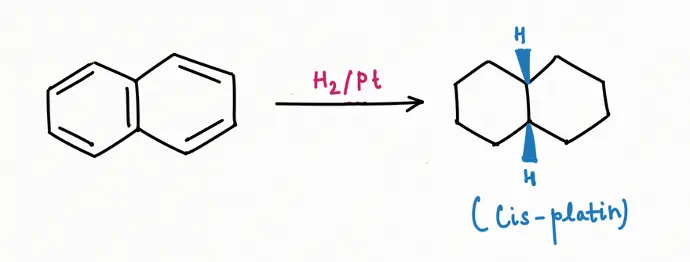
Oxidation Reaction
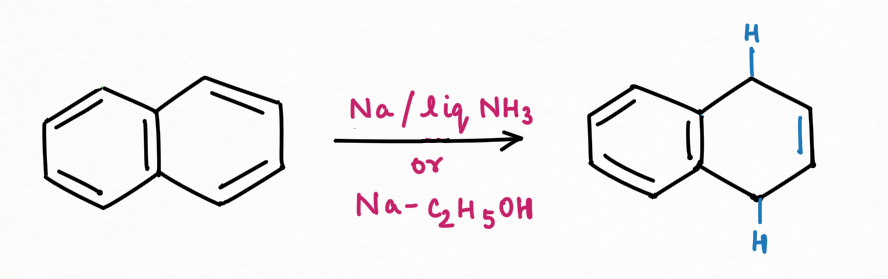
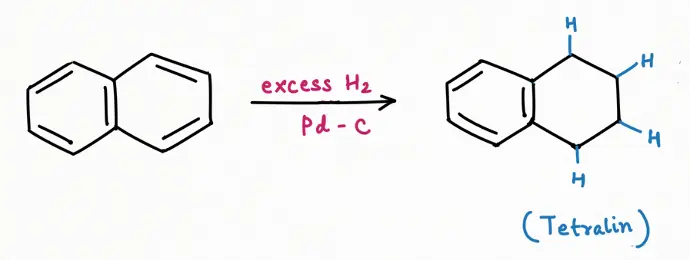
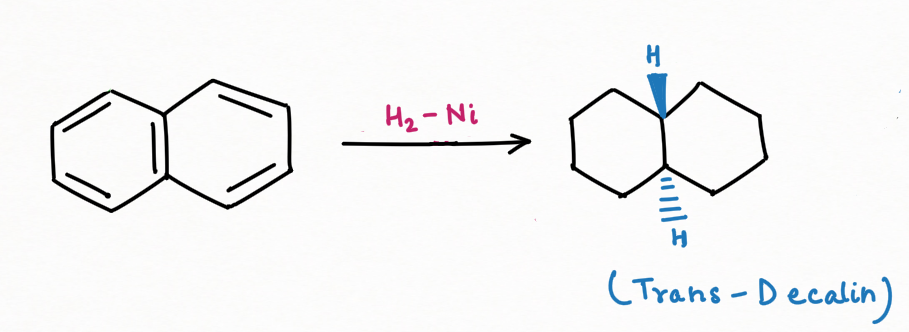
Reduction Reaction
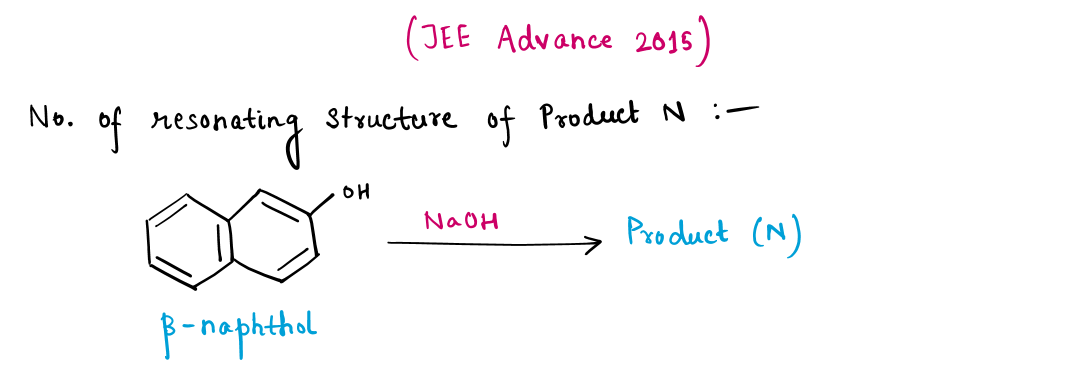
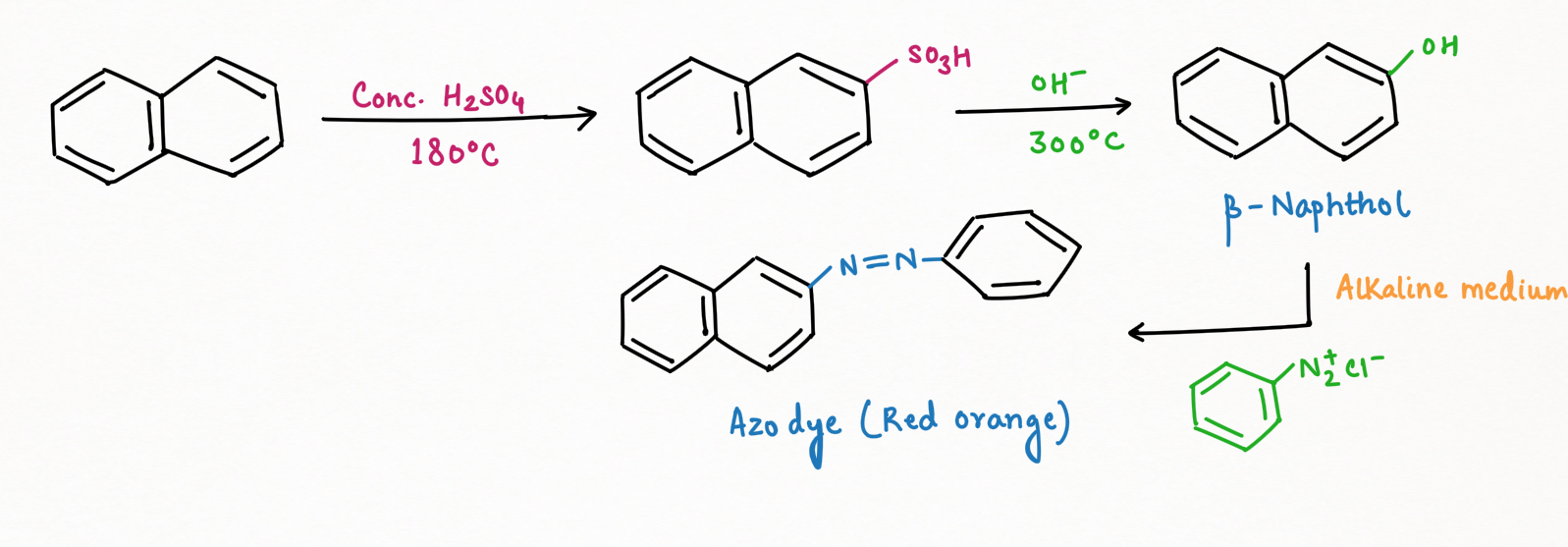
β-Naphthol Test
Resonance energy and Reactivity
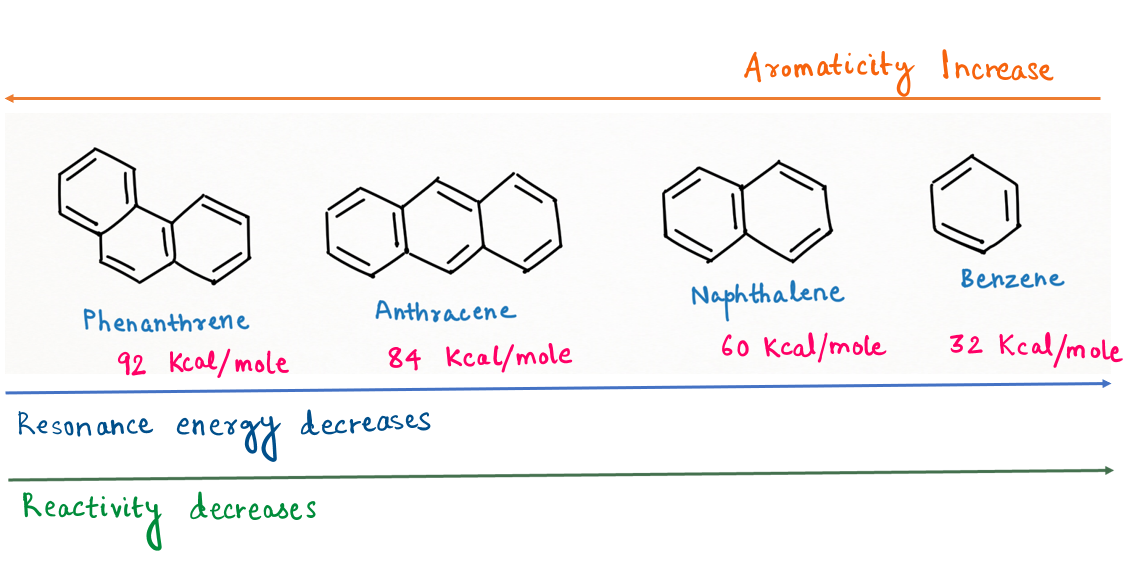
As more resonating structures, the higher the aromaticity, ultimately leads to higher resonance energy.
So, the order of resonance energy will be :
Phenanthrene (92 Kcal/mol) > Anthracene (84 Kcal/mol) > Naphthalene (61 Kcal/mol) > Benzene (32 Kcal/mol)
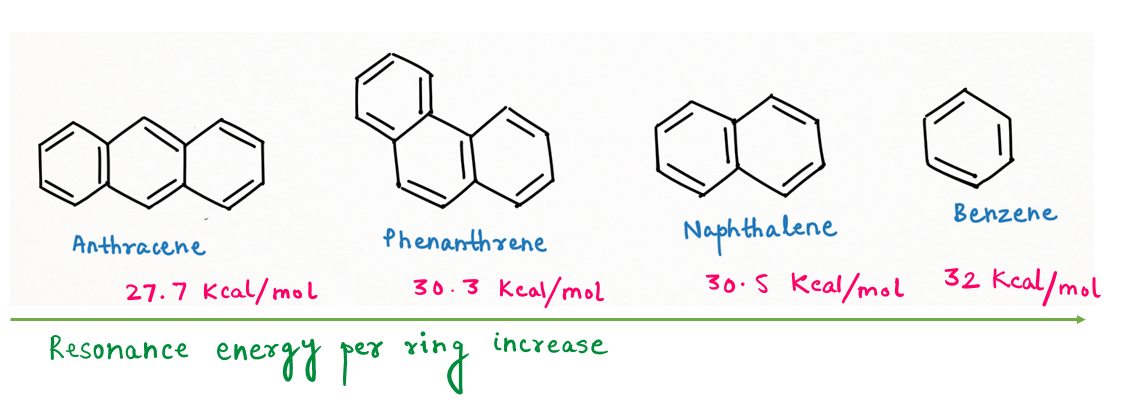
While we jot down the order for resonance energy per ring, so we see an opposite comparison to what we have seen above.
Benzene (32 Kcal/mol) > Naphthalene (30.5 Kcal/mol)> Phenanthrene (30.3 Kcal/mol) > Anthracene (27.7 Kcal/mol)
Now, let's talk about the reactivity comparison of the above four compounds especially for the electrophilic aromatic substitution reaction (EAS).
So, it is obvious to predict reactivity order from the data of resonance energy. So, naphthalene is more reactive than benzene towards EAS.
Physical properties and method of separation
- Naphthalene forms an Ideal solution with benzene. (Ideal solutions are those solutions that follow Raoult's law)
- Used as Moth Balls. In the preservation of clothes and toilet.
- Sublimes easily. Hence, it is easy to separate naphthalene from mixtures like Naphthalene-Sand/ Chalk.

4. It is not soluble even in hot water. Hence, naphthalene is separated from benzoic acid in a mixture by the method of crystallization from hot water as benzoic acid is soluble in water.
Conclusion
The blog on Science Mania provides a comprehensive overview of naphthalene, including its appearance, IUPAC naming, degree of unsaturation, and historical discovery. It details the structure, bond lengths, and resonating structures of naphthalene, explaining electrophilic substitution reactions, oxidation, and reduction reactions. The blog also covers the β-naphthol test, resonance energy, reactivity, physical properties, and methods of separation. The conclusion emphasizes the compound’s chemical significance, and a section for practice questions is included.
For more details, you can visit the full blog here.